Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Materials
Synthetic melanins display tunable properties
New method yields bioinspired polymers with UV-absorbing and charge-storing abilities
by Stu Borman
June 8, 2017
| A version of this story appeared in
Volume 95, Issue 24

Melanins have many important jobs in microorganisms, plants, and animals, including people. The cross-linked polymer pigments give color to skin, hair, and eyes, provide structural support in tissues, and protect cells from ultraviolet radiation and free-radical damage.
Organisms make melanins by using the enzyme tyrosinase to oxidize and polymerize the amino acid tyrosine, but the exact biosynthetic pathways involved are not well understood. To try to produce materials with abilities similar to those of melanin, researchers have polymerized dopamine and other tyrosine derivatives. But these syntheses have proved difficult to control, yielding products with disordered structures, poorly defined chemical and structural properties, and limited commercial applications.
Rein V. Ulijn of the City University of New York’s Advanced Science Research Center and coworkers have now developed a method for synthesizing customized melanin-like polymers controllably (Science 2017, DOI: 10.1126/science.aal5005). The customized polymers could have cosmetic, sensor, coating, and other applications.
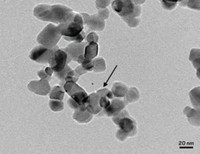
The team heats and then cools solutions of tyrosine-containing tripeptides, causing them to self-assemble into supramolecular structures, such as nanofibrils and crystals, depending on the peptide’s amino acid sequence. As the peptides self-assemble, the tyrosines interact with other tyrosines noncovalently, in ways that vary with sequence. The researchers then treat the supramolecular assemblies with tyrosinase, which oxidizes and polymerizes them into different melanin-like polymers.

Ulijn and coworkers synthesized six tripeptide sequences with one amino acid each of tyrosine (Y); phenylalanine (F), a nonpolar residue that promotes aggregation; and aspartate (D), a charged residue that enhances solubility. The tripeptides self-assemble into soluble aggregates (FDY and YDF), amorphous aggregates (FYD), nanofibrils (YFD and DFY), or crystals (DYF).
Enzymatic oxidation and polymerization of each assembly produces a distinct corresponding polymer. For instance, the FYD peptide forms a beige, randomly shaped polymer that absorbs about three times as much UV-visible light as the other polymers. DFY forms a brown-black, sheetlike polymer with about three times as much charge storage capacity as the others. And DYF forms red-brown, spherical polymer particles with better water solubility than the others.
“To the best of my knowledge, this study is the first to use tripeptides for producing synthetic melanins,” comments postdoc Chun-Teh Chen, a melanin specialist in Markus J. Buehler’s group at MIT. The technique is “a new direction in the synthesis of melanin-like materials with tunable properties.”
Ulijn says the team has filed patent applications for the process and is talking with cosmetic companies about using the materials in products that match skin tones while protecting against UV radiation and free-radical damage. “The use of simple combinations of amino acids ensures low regulatory barriers of these materials, so we believe that real-world applications may not be too far off,” he says.


Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter