Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Synthesis
Chemists discover biology’s version of the Friedel-Crafts alkylation
Newly detected enzyme alkylates aromatic rings during biosynthesis of cylindrocyclophanes
by Bethany Halford
June 29, 2017
| A version of this story appeared in
Volume 95, Issue 27

By chemistry standards, the Friedel-Crafts alkylation is a venerable reaction. First reported by chemists Charles Friedel and James Crafts in 1877, the reaction attaches an alkyl substituent to an aromatic ring using an alkyl halide as one of the reactants. It turns out that Nature has been doing this reaction for even longer—scientists just didn’t know it until now.
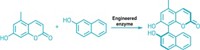
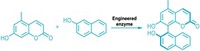
Harvard University’s Emily P. Balskus, Hitomi Nakamura, and Erica E. Schultz have discovered an enzyme, dubbed CylK, that accomplishes this same transformation during the biosynthesis of cylindrocyclophanes, natural products made by cyanobacteria that are toxic to cells (Nat. Chem. Bio. 2017, DOI: 10.1038/nchembio.2421). “Because aromatic-alkyl linkages are such important motifs in synthetic compounds, we’re hoping that the enzyme we found will ultimately be useful for chemical production,” says Balskus, who led the research effort.
Her team also found a new type of metal-dependent halogenating enzyme, named CylC, that creates secondary alkyl chlorides. Secondary alkyl halides can be tricky to prepare in the lab because the intermediates en route to making them tend to rearrange. The same can be said for using secondary alkyl halides as reactants in the Friedel-Crafts alkylation.
“Secondary alkyl halides have been notoriously problematic substrates for that reaction,” Balskus points out. “They’re not very electrophilic, and because the Friedel-Crafts alkylation involves generating a carbocation intermediate, you get a lot of competing side reactions and rearrangements,” she says. “If we can engineer these enzymes to accept substrates that would be useful building blocks for chemical synthesis, they would fill gaps in our synthetic chemistry toolbox.”
Next, Balskus hopes to pin down the mechanisms of the two novel enzymes discovered by her group. X-ray crystal structures of CylC and CylK with bound substrates could be particularly informative, she notes.
Balskus and coworkers “have performed an outstanding study—remarkable in its breadth, notable in the novelty of biochemistry encountered, and impactful for its importance to understanding cyanobacterial secondary metabolism as well as biotechnological applications,” comments William Gerwick, an expert in the biosynthesis of marine natural products at Scripps Institution of Oceanography.
“While much work remains to further characterize both reactions, the current studies place these on a solid biosynthetic foundation,” Gerwick continues. This is important, he adds because an informatics analysis of available sequenced cyanobacterial genomes suggests the halogenation to be a highly prevalent mechanism in these organisms and underpins their potential use in biocatalysis applications.


Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter