Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Biological Chemistry
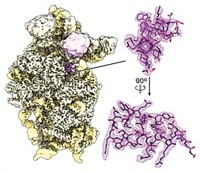
The first herpes capsid at atomic resolution
At 3.9 Å resolution, structure reveals how human cytomegalovirus capsids withstand pressure caused by packing in loads of DNA
by Sarah Everts
June 29, 2017
| A version of this story appeared in
Volume 95, Issue 27
A structure of the herpes virus capsid reveals atomic-level details for the first time thanks to cryo-electron microscopy. A team led by Hong Zhou at the University of California, Los Angeles, focused in on the biggest of all herpes pathogens, human cytomegalovirus, whose 1300-Å-diameter capsid features some 4000 proteins (Science, 2017, DOI: 10.1126/science.aam6892). Human cytomegalovirus is responsible for birth defects, organ transplant infections, and AIDS deaths. Its 235-kilobase DNA genome is more than 50% larger than that of the cold sore–causing herpes simplex virus 1. Packing in all that DNA produces tens of atmospheres of pressure that the capsid architecture must withstand. For example, a chassis protein forms a net-like layer surrounding the capsid, thereby increasing structural stability. “Our structure should inform rational design of countermeasures against human cytomegalovirus, other herpes viruses, and even HIV/AIDS,” the researchers note.



Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter