Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Biological Chemistry
Potential tuberculosis fighter takes an unusual tack
Small molecule could attack bacterium by stabilizing key metabolic enzyme, a strategy that would be difficult for the bacterium to circumvent
by Bethany Halford
July 10, 2017
| A version of this story appeared in
Volume 95, Issue 28
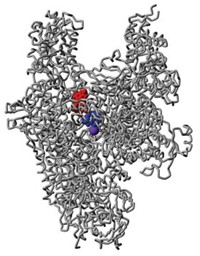
The bacterium Mycobacterium tuberculosis claimed 1.8 million lives worldwide in 2015, according to the World Health Organization, making tuberculosis the deadliest infectious disease on Earth. Scientists led by Deborah T. Hung of Broad Institute report a small molecule, called BRD4592, that inhibits one of the bacterium’s key metabolic enzymes—tryptophan synthase (Nat. Chem. Biol. 2017, DOI: 10.1038/nchembio.2420). BRD4592 shuts down this enzyme, not by binding to its active sites, but by binding in the channel between the enzyme’s subunits. This allosteric binding prevents the enzyme from completing the cycle it uses to make tryptophan. It also blocks indole, an important intermediate en route to tryptophan, from shuttling between the enzyme’s two active sites. Hung’s team believes this is one of the most complicated allosteric inhibitors reported to date. The scientists found that mice metabolized BRD4592 too quickly to evaluate the compound’s efficacy in infected animals, but the team did determine that M. tuberculosis engineered without a tryptophan synthase enzyme were unable to establish an infection in mice. This, Hung’s team believes, makes allosteric inhibition of this enzyme an attractive strategy for fighting tuberculosis.


Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter