Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Energy
Tracking lithium ions in an electrolyte
A fluorescent imaging agent that binds directly to lithium ions could help researchers better understand why batteries wear out
by Katherine Bourzac, special to C&EN
August 7, 2017
| A version of this story appeared in
Volume 95, Issue 32

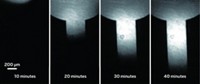
Over time, lithium-ion batteries need more frequent recharging. And on rare occasions, the batteries can catch fire when lithium builds up on the anode, forming sharp spikes that pierce the membrane that separates the anode from the cathode. A new way to directly image lithium ions could help researchers better understand why these problems happen (ACS Sens. 2017, DOI: 10.1021/acssensors.7b00087). Randall H. Goldsmith of the University of Wisconsin, Madison, and colleagues redesigned a fluorescent organic molecule that binds selectively to lithium ions so that the molecule fluoresces when excited by blue light instead of ultraviolet light, which can reduce image quality. The researchers then created a simple test platform consisting of the molecule—2-(2-hydroxyphenyl)naphthoxazole (shown)—and an electrolyte within a microfluidic channel. They injected lithium chloride solutions into the channel and showed that the fluorescence intensity grew with increasing ion concentration. Then the group used the system to track the diffusion of lithium through the electrolyte. They now plan to design a test battery that’s transparent on one side to evaluate the fluorescence imaging method further.


Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter