Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Materials
Flexible batteries get safer with sodium-based aqueous electrolytes
Devices could power wearable and implantable medical devices with reduced chemical risk
by Stu Borman
August 20, 2017
| A version of this story appeared in
Volume 95, Issue 33
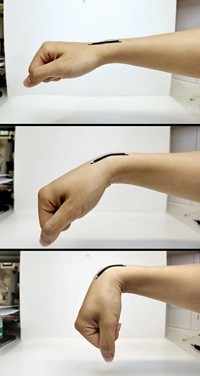
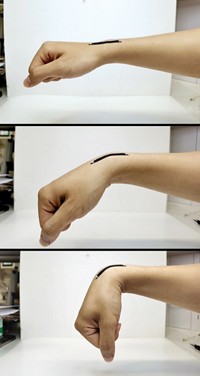
Researchers are developing batteries and energy-storing supercapacitors that flex with the body while powering wearable and implantable medical devices. But so far these power sources have used electrolytes containing strong acids, strong bases, or toxic and flammable organic solutions, which can cause harm if they leak. Yonggang Wang, Huisheng Peng, and coworkers at Fudan University have now created bendable sodium-ion batteries containing biofriendly aqueous electrolytes (Chem 2017, DOI: 10.1016/j.chempr.2017.05.004). The batteries have a Na0.44MnO2 cathode and a carbon-coated NaTi2(PO4)3 anode in contact with one of three sodium-based electrolytes: a sodium sulfate solution, intravenous saline, or a cell culture medium. In a belt-shaped design, the cathode and anode sandwich an electrolyte-soaked separator. And a fiber-shaped design has carbon nanotube electrodes embedded with cathode and anode nanomaterials and surrounded by electrolyte. The batteries have charge capacities and power outputs per unit volume comparable to those of previously reported flexible lithium-ion batteries and supercapacitors. But for now their power output per unit mass and maximum operating voltage are too low to be commercially practical. In future work, the researchers hope to improve the batteries by using higher capacity electrode materials and to test their ability to energize actual medical devices.



Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter