Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Synthesis
Reaction plays favorites in polyols
ACS meeting news: Ruthenium-catalyzed process zeroes in on individual hydroxyls in multi-hydroxylated compounds
by Stu Borman
August 23, 2017

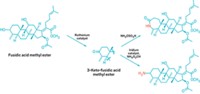
Chemists would like to be able to modify compounds containing multiple hydroxyl groups, such as the toxin ouabain and the antiparasitic drug ivermectin, to generate new molecules with diverse uses in medicine, molecular biology, and agroscience. One way to do this would be to selectively oxidize individual hydroxyls to ketones, which are synthetically versatile groups in that they can be readily converted to nitrogen-based groups, such as oximes and amines, or can be leveraged to add adjacent groups in a molecule’s carbon framework.
A team of researchers at the University of California, Berkeley, has now designed a catalyst capable of such a feat.
Dehydrogenating selected hydroxyls to ketones in multi-hydroxylated compounds known as polyols has been difficult. A common strategy is to add protecting groups to the hydroxyls you don’t want to react and then deprotect them later. But this is laborious, requiring multiple reactions instead of just one. Although a few synthetic procedures can oxidize specific hydroxyls in polyols to ketones, they generally cause undesirable side reactions or are poorly selective for specific hydroxyls, producing complex mixtures instead of relatively pure ketone products.
John F. Hartwig and Christopher K. Hill of UC Berkeley speculated that one way to improve selectivity would be to develop a catalyst that oxidizes polyol secondary alcohol groups—hydroxyls on carbon atoms that are bonded to two other carbon centers—much more readily than primary alcohol groups—hydroxyls on carbons connected to only a single carbon center.
The catalyst they designed has strongly electron-donating phosphine ligands that make its ruthenium center electron-rich, weakening its oxidizing powers. That makes the catalyst selective for secondary hydroxyls, which are more electron-rich than primary hydroxyls and thus better substrates for a weakly oxidizing catalyst.
Hartwig and Hill demonstrated the catalyst’s power and versatility by using it to selectively oxidize a single hydroxyl group in more than a dozen polyol natural products and using subsequent catalytic reactions to convert the resulting ketones into nitrogen-modified products. They recently reported the work in Nature Chemistry (2017, DOI: 10.1038/nchem.2835) and presented it this week during the ACS national meeting in Washington, D.C., during a session sponsored by the Catalysis Science & Technology Division.
The researchers developed a set of rules that predict which hydroxyls in a polyol the catalyst will oxidize selectively. In general, the catalyst is selective for electron-rich, sterically hindered secondary hydroxyls. The reaction does not produce dione products by oxidizing multiple hydroxyls in the same compound, owing to the catalyst’s high sensitivity for electronic and steric differences in the properties of different hydroxyls.
The new selective alcohol dehydrogenation reaction “has many advantages over traditional methods, including lower catalyst loading, milder conditions, higher yields, and improved selectivity,” says transition-metal catalysis specialist Guangbin Dong of the University of Chicago. “I am most impressed by the reaction’s unusual chemoselectivity in the dehydrogenation of secondary hydroxyls. Without doubt, this technology is going to be highly useful for medicinal chemistry.”
The technique represents “a very important step forward in the ability to do catalytic, site-selective alterations of complex molecules,” says Scott Miller of Yale University, an expert on site-selective catalysts. It will be useful for achieving selectivity in the conversion of complex starting materials to bioactive analogs, he says.



Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter