Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Materials
Nanodiamonds reduce short-circuit risk in rechargeable lithium batteries
Carbon crystals prevent formation of needelike dendrites during charging cycles
by Mitch Jacoby
September 4, 2017
| A version of this story appeared in
Volume 95, Issue 35

Adding a small amount of nanodiamonds to the electrolyte solution in lithium batteries can make these popular energy storage devices safer, according to a study (Nat. Commun. 2017, DOI: 10.1038/s41467-017-00519-2).
As lithium batteries are charged, lithium ions in the electrolyte solution migrate from the cathode through a micrometer-thin porous polymer separator and insert themselves in the anode. The anode in lithium-ion batteries is generally a form of graphite. That material is used because it reduces the chance that tiny, needlelike lithium structures—dendrites—will catastrophically short-circuit the battery by growing uncontrollably from the anode, piercing the insulating separator, and contacting the cathode.
If instead of using graphite, the anode were pure lithium metal, the battery’s charge-storage capacity could be almost 10 times as large. But metallic lithium anodes exacerbate the dendrite problem.
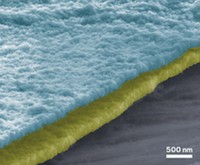
A team led by Drexel University’s Yury Gogotsi and Qiang Zhang of Tsinghua University reports that low-cost nanodiamond particles in the electrolyte solution guide lithium during electrochemical deposition to form smooth, dendrite-free films on lithium and other metallic electrodes.
The researchers explain that the nanodiamond surfaces serve as energetically favorable Li-ion adsorption and nucleation sites that enable Li ions to easily diffuse across the surface, coating it uniformly, rather than accumulating in small spots and forming dendrites.



Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter