Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Business
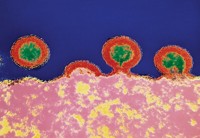
Sanofi ends Zika vaccine research
Other vaccine efforts continue, even as virus abates
by Lisa M. Jarvis
September 11, 2017
| A version of this story appeared in
Volume 95, Issue 36

Citing reduced funding from the U.S. Biomedical Advanced Research & Development Authority (BARDA), drugmaker Sanofi is shutting down development of its vaccine for Zika virus.
Last year, BARDA committed $43.2 million to support the manufacture and Phase II clinical study of Sanofi’s Zika vaccine. Now, the government organization is limiting its contract with Sanofi to a Zika surveillance study, the results of which could benefit all vaccines in the pipeline. Although Sanofi will no longer pursue the Phase II study, the goal is to get the project to a point where it can be easily restarted if Zika roars back.
Zika was declared a public health emergency by the World Health Organization in early 2016 after several thousand Brazilian women infected during a spring 2015 outbreak gave birth to infants with microcephaly—a rare condition that causes babies to have smaller-than-expected heads and brains.
Since that time, industry, academic, and government researchers have been trying to understand the virus and develop drugs, diagnostics, and vaccines against it.
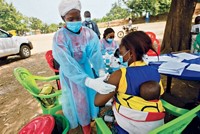
Clinical trials of several other Zika vaccines continue. But even as infectious disease experts laud the speed at which those products are moving toward commercialization, they warn that vaccines are not a panacea for a virus like Zika.
“One of the things we’ve learned in recent years with Zika, dengue, and West Nile is there are no easy solutions for these ecologically complex mosquito-borne arbovirus diseases,” says David M. Morens, senior adviser to the director of the National Institute of Allergy & Infectious Diseases.
The problem, Morens explains, is that vaccines are helpful for controlling major outbreaks, but viruses tend to flare up, peter out, and then pop up again in small, unpredictable clusters. In Puerto Rico, the hardest-hit U.S. territory, new cases of Zika dropped from nearly 35,000 in 2016 to less than 500 so far this year. Although everyone is happy to see the virus abate, it leaves public health officials with a quandary: Who, if anyone, should be vaccinated?
Still, Morens is encouraged by the number of Zika vaccines in the pipeline. “Show me a time when we’ve done so much in such a short period of time for any disease—I just can’t think of any,” he says.



Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter