Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Synthesis
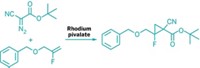
Fluorine flow chemistry yields flucytosine
Streamlined synthesis offers better access to an essential drug for treating HIV/AIDS-related fungal infections
by Stephen K. Ritter
January 23, 2017
| A version of this story appeared in
Volume 95, Issue 4
In Africa, about one-third of the roughly 2 million HIV/AIDS-related deaths each year stem from the fungal infection cryptococcal meningitis. The first-line treatment for this disease and other HIV/AIDS-related fungal infections is a combination of flucytosine and amphotericin B. Although these two drugs are on the World Health Organization’s List of Essential Medicines, uneven global pricing and distribution has left flucytosine largely unavailable to Africans who need it—flucytosine is relatively expensive to make and there’s little generic competition to reduce cost. Answering a call from the infectious diseases community, a team led by Graham Sandford of Durham University, working in collaboration with scientists at Sanofi Aventis and the nonprofit group MEPI within the EU’s Innovative Medicines Initiative Chem21 network, has developed a streamlined method for making flucytosine that could improve its global availability (Org. Process Res. Dev. 2017, DOI: 10.1021/acs.oprd.6b00420). Flucytosine is currently manufactured in a four-step process starting from uracil, which includes fluorination, chlorination, amination, and hydrolysis steps. Taking advantage of the Durham group’s expertise in selective fluorinations using flow reactors, the team developed a pilot-scale continuous-flow process to convert cytosine directly into flucytosine at a rate of 60 g per hour. Besides improving access to flucytosine to combat fungal infections, the researchers believe the new process could benefit flucytosine’s use as an intermediate in the synthesis of anticancer and HIV drugs.



Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter