Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Materials
Chemists triple down on metal helicates
Combining a multidentate ligand with various metals leads to triple-stranded complexes with potentially useful magnetic properties
by Stephen K. Ritter
October 9, 2017
| A version of this story appeared in
Volume 95, Issue 40
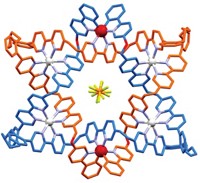
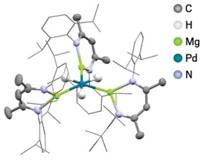
Chemists have come far in using metal-assisted self-assembly of organic building blocks to construct molecules with three-dimensional architectures. For example, a team led by supramolecular chemistry specialists Pradyut Ghosh of the Indian Association for the Cultivation of Science and Christoph A. Schalley of Free University of Berlin has been matching various metal salts with a multidentate ligand it developed that is made up of a 2,2'-bipyridine core flanked by triazolyl-pyridine groups. This combination leads to formation of unusual trimetallic triple-stranded helicate complexes, (L3M3)6+, where L is the ligand and M is Fe, Co, Ni, Cu, or Zn or a combination of two of the metals. In continuing the work, the team has now systematically used electrospray ionization mass spectrometry to study the formation mechanism and thermodynamic stabilities of the helicates when mixtures of metals are used. The researchers have found that some metals outcompete others for binding sites, leading some of the helicates to be interconverted into others. In one case, they observed formation of (L3FeZnCu)6+ (shown), the first example of such a complex containing three different metals. However, this trinuclear species only appears as an intermediate on the way to (L3Cu3)6+, which is the most stable isolable helicate (Inorg. Chem. 2017, DOI: 10.1021/acs.inorgchem.7b01980). Ghosh and Schalley say the magnetic properties of the trimetallic helicates will be of interest for developing smart materials, in particular for the versions having a combination of diamagnetic and paramagnetic metals.


Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter