Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Synthesis
Quick and easy acyl fluorides
Bench-stable trifluoromethylthiolate salt simplifies functionalization of carboxylic acids
by Stephen K. Ritter
October 30, 2017
| A version of this story appeared in
Volume 95, Issue 43

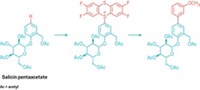
Tetramethylammonium trifluoromethylthiolate, [N(CH3)4]SCF3, is a mouthful to say, but the inexpensive, bench-stable, and easy-to-use salt has been impressing chemists over the past few years for its versatility in adding SCF3 groups to molecules and as a fluorinating reagent. For example, Franziska Schoenebeck and her group at RWTH Aachen University have shown that [N(CH3)4]SCF3 has untapped potential as a fluorinated surrogate of thiophosgene (SCCl2), using it to functionalize alcohols and amines. Going further, Schoenebeck’s group has now used the trifluoromethylthiolate salt to convert aromatic and aliphatic carboxylic acids to acyl fluorides, which themselves are useful reagents (Org. Lett. 2017, DOI: 10.1021/acs.orglett.7b02516). The appeal of using [N(CH3)4]SCF3 to fluorinate carboxylic acids is its ability to replace specialized reagents derived from SF4. Those reagents often are toxic and corrosive, require additives, and tend to have poor functional-group tolerance and selectivity leading to undesired by-products. The trifluoromethylthiolate salt, first reported years ago but little used until recently, does away with those problems. Schoenebeck says her team has had “quite a lot of interest” from industry and other academic groups about using [N(CH3)4]SCF3, which could lead to efforts by her group to commercialize it.


Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter