Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Analytical Chemistry
Method reveals secrets of bimetallic catalysts
Single-molecule, single-particle imaging study quantifies catalytic enhancement
by Mitch Jacoby
November 27, 2017
| A version of this story appeared in
Volume 95, Issue 47
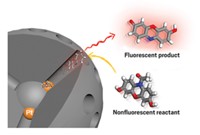
Nanoparticle catalysts containing two types of metals play an important role in petrochemical reforming, hydrogenation, and other industrial processes. Researchers have long known that the combination of metals leads to enhanced catalytic activity compared with the corresponding monometallic catalysts. Knowing the molecular level details that underpin the bimetallic enhancement could lead to even better catalysts—ones that use less energy and generate fewer by-products. But because of a lack of nanoparticle uniformity and difficulty tracking reactions on the nanometer scale, those details have remained hidden. Now they’re being exposed. Peng Chen of Cornell University and coworkers prepared palladium nanorods tipped with a gold sphere and used the catalysts to mediate a model photocatalytic reaction—reduction of nonfluorescent resazurin to fluorescent resorufin. By scrutinizing the system with electron microscopy and a fluorescence microscopy method that has single-molecule resolution, the team tracked thousands of reactions individually. In that way, they pinpointed with nanometer resolution, where on more than 50 particles, a reaction occurred (ACS Cent. Sci. 2017, DOI: 10.1021/acscentsci.7b00377). The data show that the nanoscale interface of the two metals is the catalytic hot spot: It’s about 50% more active than single-metal regions. Also, the most active facets of the monometallic catalysts are the best spots to form bimetallic interfaces.



Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter