Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Synthesis
Chemists forge green path to alkylated amines
Process uses microbe-derived amino acids to produce the industrially important building blocks
by Sam Lemonick
December 18, 2017
| A version of this story appeared in
Volume 95, Issue 49

Chemists use alkylated amines to build plastics, pharmaceuticals, and more. Unfortunately, making these important building blocks on a large scale is energy intensive and relies on nonrenewable feedstocks. Now a team of researchers report a green approach to synthesizing the molecules.
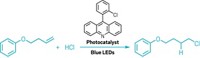
Tao Yan, Ben L. Feringa, and Katalin Barta of the University of Groningen describe an environmentally-friendly catalytic process that uses alcohols to add alkyl groups to amino acids harvested from microbes (Sci. Adv. 2017, DOI: 10.1126/sciadv.aao6494). The method retains the chirality of the amino acids and releases water as its only waste product.
Calling the research “nothing short of revolutionary,” Paul T. Anastas, the director of Yale University’s Center for Green Chemistry & Green Engineering, says the approach could mean a cheaper, cleaner way to make these industrially crucial building blocks.
Making alkylated amines is so energy intensive because it requires the Haber-Bosch process, which converts atmospheric nitrogen to ammonia at around 500 °C. To add alkyl substituents to ammonia, chemists use molecules derived from fossil fuels and reactions that often generate as much waste as they do useful products.
Yan, Feringa—who shared the 2016 Nobel Prize in Chemistry—and Barta instead let nature do the hard work of reducing nitrogen: They isolated amino acids from bacteria. As for adding alkyl substituents to these amino acids, ethanol, isopropanol, and other simple alcohols act as both solvents and reactants. The chemists initially used a ruthenium catalyst but also demonstrated the reaction with a catalyst containing iron, a more abundant metal.
In either case, the catalyst borrows a hydrogen atom from the alcohol and produces a carbonyl intermediate that then reacts with the amino acid, shedding a water molecule. The resulting imine intermediate then takes a hydrogen back from the catalyst, producing an alkylated amine.
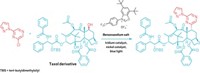
The researchers demonstrated their method by synthesizing a surfactant from glycine and 1-dodecanol using an iron catalyst. Feringa says they believe the technique has broad potential beyond surfactants. The chemists have filed for a patent on the method and are looking for partners to explore adapting it for industrial uses.
“This shows, once again, that green chemistry is just simply better chemistry,” Anastas says.



Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter