Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Pharmaceuticals
Making the diabetes drug exenatide more stable
Changing the delivery system and swapping one amino acid in the protein drug could reduce its injection frequency
by Katharine Gammon
August 30, 2017

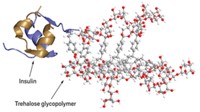
Exenatide, a widely used diabetes drug, requires that patients give themselves a subcutaneous injection at least once per week. But because of a new development, less frequent injections might soon be possible. By tweaking one amino acid of exenatide and attaching it to hydrogel microspheres, researchers have developed a longer-acting version and tested it in rodents (ACS Chem. Biol. 2017, DOI: 10.1021/acschembio.7b00218). The new drug could lead to monthly injections instead, the researchers report.
Exenatide— a member of a drug class known as glucagon-like peptide 1 (GLP-1) receptor agonists—was inspired by a surprising source: a peptide first isolated from Gila monster spit. In the presence of glucose, these drugs stimulate insulin secretion, which in turn lowers excess glucose. They also lower appetite and cause weight loss. Most recently, exenatide has slowed the progression of Parkinson’s disease.
To reduce the number of injections needed, Daniel V. Santi, a pharmaceutical chemist at the University of California, San Francisco, and his colleagues wanted to extend the half-life of the drug. They started by using a strategy that they’d previously developed: They attached exenatide to hydrogel microspheres using a pH-dependent linker designed to slowly release the drug over a one-month period.
But in animal tests, the tethered drug’s half-life topped out at two weeks. After analyzing the drug’s structure, the researchers noticed a two-amino acid sequence, an asparagine followed by a glycine, that commonly degrades when the asparagine loses its amide group and rearranges. In exenatide, this deamidation occurred under physiological conditions within a couple of weeks. By changing asparagine to glutamine—which is less prone to deamidation—the researchers stabilized the structure.
“It’s the smallest possible change you can make to a peptide,” explains Santi. By extending the amino acid sidechain, the team lengthened the half-life of degradation from about two weeks to over six months.
As a bonus, the more stable version’s behavior in the body is indistinguishable from exenatide in animal trials. In tests of the modified exenatide attached to the hydrogel microspheres, the drug had a half-life of one month in obese, diabetic rats. If injected twice monthly, the blood levels of the active drug were indistinguishable from those in rats who had been continuously infused. Because exenatide has undergone extensive clinical studies, Santi expects to be able to move this modified drug into clinical testing soon.
The more stable exenatide-hydrogel combination could lower costs to patients. In theory the technology could also produce other long-acting drugs, depending on the potency and stability of the original molecule, says Santi. He cofounded the biotech company ProLynx that is developing this modified exenatide.
Lalita Prasad-Reddy, a clinical pharmacist at Chicago State University, says a monthly formulation of a GLP-1 drug would reduce the medication burden for patients and could solve some persistent problems with current injectables. Currently the peaks and troughs of GLP-1 concentrations with daily or weekly injections can lead to nausea and vomiting in some patients. She points out that a monthly formulation could potentially reduce those side effects.



Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter