Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Safety
U.S. FDA halts use of triclosan in health care antiseptics
Agency defers action on two quaternary ammonium compounds
by Cheryl Hogue
December 22, 2017
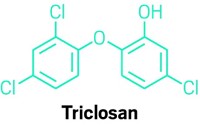
The antibacterial compound triclosan, already banned in the U.S. from consumer soaps, will no longer be allowed in antiseptic products used in hospitals and other health care settings. The U.S. Food & Drug Administration on Dec. 20 deemed triclosan and 23 other antiseptic ingredients to not be generally recognized as safe and effective.
“There was a lack of sufficient safety and efficacy data” for the 24 affected chemicals, explains FDA Commissioner Scott Gottlieb.
The new regulation affects hand washes and rubs used by health care professionals, surgical hand scrubs and rubs, and antiseptic preparations used on patients’ skin before injections or surgery.
FDA’s action stems from a settlement the agency made with the Natural Resources Defense Council (NRDC). The environmental group sued the agency in 2010 for failing to finalize a 1978 proposal to ban triclosan in soaps.
In the new regulation, FDA put off deciding whether six other ingredients are safe and effective in antiseptic health care products, giving manufacturers more time to provide data to the agency. These substances are: two quaternary ammonium compounds, benzalkonium chloride and benzethonium chloride; chloroxylenol; ethyl alcohol; isopropyl alcohol; and povidone-iodine.
The American Cleaning Institute, which represents producers of soaps and detergents, is pleased that the agency deferred action on the six chemicals.
“Manufacturers need sufficient time to provide FDA with additional safety and efficacy data to support the continued use of these products in health care facilities,” the industry group says. “The active ingredients used in health care antiseptic drug products have very favorable benefit/risk ratios demonstrated over many years of extensive use.”
But Mae Wu, a senior attorney with NRDC, is concerned about the delay. Companies have had decades to provide FDA with the safety and efficacy data, she points out.
NRDC is particularly focused on the two quaternary ammonium compounds, saying the data suggests that these chemicals may cause health problems. Hand sanitizers containing these chemicals are marketed as alcohol-free and are widely used in schools as well as in health care settings, Wu says.
Wu is worried about how long FDA will take to make a determination about the six antibacterial substances.
“I don’t want us to wait another 40 years for FDA to make a decision on these other chemicals,” she says, noting the time that elapsed between FDA’s proposal to ban triclosan and its final action.
FDA banned triclosan and certain other antiseptic chemicals in consumer soaps and body washes more than a year ago. It found that companies haven’t shown the compounds to be safe for long-term daily use, and said that manufacturers failed to show that products with the substances were more effective at preventing the spread of germs than plain soap and water. FDA also cited concern about potential hormonal effects and antibiotic resistance associated with the chemicals.




Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter