Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Synthesis
3-D-printed reactors construct complex small molecules
Approach aims to standardize chemical synthesis so anyone can do it
by Bethany Halford
January 18, 2018
| A version of this story appeared in
Volume 96, Issue 4
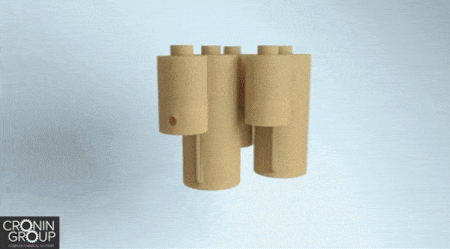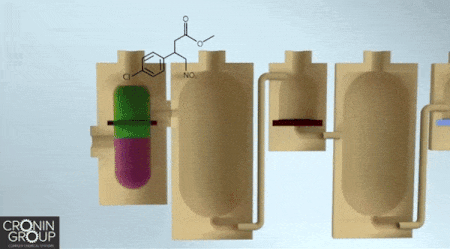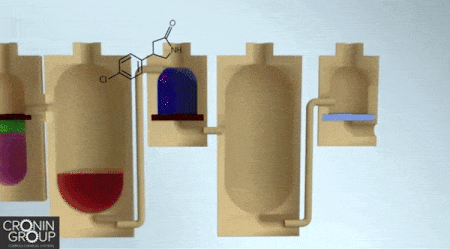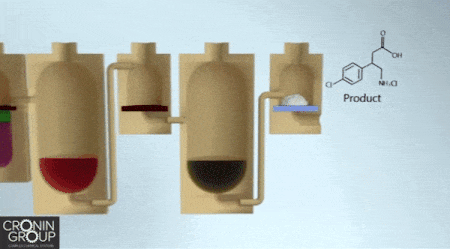

Constructing complex chemicals could one day be as easy as building an Ikea bookshelf, if a synthesis approach developed by chemists at the University of Glasgow catches on. Instead of relying on a hex key as the central tool in the assembly process, molecule makers would use a three-dimensional printer. Leroy Cronin and colleagues report a chemical manufacturing process that uses customized, 3-D-printed, sealed reactors that users load with chemicals according to simple instructions.
“The aim is to make a molecule with very little expertise just following the instruction manual,” Cronin explains. He thinks this digitized chemistry technology will allow nonchemists, such as biologists and medical professionals, to synthesize molecules with short shelf lives when and where they’re needed. It would also create a method for reproducibly synthesizing a compound. “Once you’ve digitized the process to do the synthesis, it can be resurrected forever,” he says.

As a proof of concept, Cronin’s team designed a 3-D-printed polypropylene reactor with multiple compartments to synthesize the muscle relaxant baclofen. This particular reactor performs three reactions, two liquid-liquid extractions, and a set of evaporations and filtrations, ultimately producing baclofen hydrochloride as a white powder. The entire process can be described through a platform-independent code so that anyone could print the reactor and duplicate the synthesis (Science 2018, DOI: 10.1126/science.aao3466).
“We wanted to come up with a way of ensuring if you put chemicals in a reactor system that they would process the same way each time, giving you a complex reaction procedure without anything going wrong,” Cronin says. The concept is to take the complex orchestration of reactants, substrates, and techniques performed by laboratory chemists and put those in the design of a reactor that could be used by anyone.
“That’s a better use of the chemist’s time,” Cronin says. “Organic chemists are creative individuals that are highly skilled. Why should they be enslaved to make the same molecule again and again?”
“The concept of digitizing chemistry is appealing,” comments Rob Ameloot, an expert in 3-D printing at KU Leuven. “What I find particularly tempting are the fast iteration cycles made possible by the combination of computer-controlled design and 3-D printing.” Ameloot notes that while Cronin has shown a nice synthesis of an active pharmaceutical ingredient (API), it’s not the same as making a formulated drug, which includes nonactive substances that tune a drug’s release.
Cronin says he doesn’t intend for digitized chemical synthesis to replace large-scale API manufacturing. However, he notes, it could be used for small, bespoke applications, for people to perform chemical synthesis uniformly, and to crowdsource validation, regulation, and quality control. “This is going to enable us to standardize chemical synthesis.”





Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter