Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Materials
Grain boundaries impede ion conduction in solid electrolytes
Simulations quantify performance of solid-state replacements for flammable liquids in Li-ion batteries
by Mitch Jacoby
January 22, 2018
| A version of this story appeared in
Volume 96, Issue 4

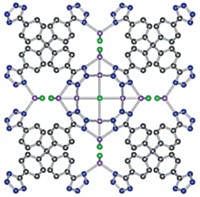
Rechargeable lithium-ion batteries power many of today’s electric vehicles and nearly all portable electronic gadgets and power tools. These devices boast extreme reliability, but they depend on a flammable liquid organic electrolyte solution to shuttle ions between the electrodes, and those liquids pose a tiny but potentially serious fire hazard. So researchers have been searching for nonflammable solid electrolytes as replacements. Although some are nearing commercialization, scientists still do not know some basics, for example, how grain boundaries—interfaces between crystallites of the electrolyte—affect lithium-ion conduction, which controls battery current. So James A. Dawson and M. Saiful Islam of the University of Bath and coworkers carried out molecular dynamics simulations to study how readily lithium ions can hop across grain boundaries in polycrystalline Li3OCl, a promising solid electrolyte candidate. The team stresses that grain boundaries may enhance or impede ion conduction: The effect cannot be determined a priori. It turns out they don’t help matters in Li3OCl. Crystal interfaces lower ion conductivity by about a factor of 10 (J. Am. Chem. Soc. 2018 DOI: 10.1021/jacs.7b10593). The team used the results to develop a model that quantifies the effect of grain boundaries on conductivity as a function of crystallite size and suggests how tailoring the microstructure can optimize the performance of a variety of solid electrolyte materials.


Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter