Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Environment
Insulin falls apart without water
Computer simulations show water holds the insulin hexamer together
by Sam Lemonick
January 29, 2018
| A version of this story appeared in
Volume 96, Issue 5
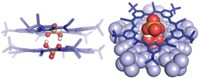
Computer modeling suggests that water molecules play a key role in stabilizing insulin for storage in the body, according to a study (J. Phys. Chem. B.2018, DOI: 10.1021/acs.jpcb.8b00453). Insulin is bioactive as a monomer, but it is stored in the pancreas in groups of six, coordinated around two zinc cations. X-ray crystallography analysis by Biman Bagchi and colleagues at the Indian Institute of Science paired with computer simulations revealed that water molecules, about 10 on average, are also present in an interior cavity of the hexamer. So they set out to determine what function, if any, these water molecules play. The group performed atomistic molecular dynamics simulations of the hexamer, zinc cations, and water molecules. They found that three water molecules and three histidine residues coordinate to each of the cations in an octahedral arrangement. Other water molecules enter and leave the cavity, but the simulation showed that these molecules stay fixed nearly in place while inside the hexamer. The study identified an average of 15 hydrogen bond interactions between the 10 water molecules in the cavity, as well as bonds to peptide residues. The group likens the water molecules to a backbone in the hexamer. Next, the researchers simulated the hexamer and zinc cations in the absence of water molecules. That led to new interactions between the cations and peptide residues, and the hexamer cavity collapsed within picoseconds. They did not test the hexamer with other cations, such as calcium, which are seen in nature. Bagchi says this new understanding of water’s role could help explain how the hexamer breaks apart to deliver monomeric insulin. It may also help protect insulin in pharmaceuticals against aggregation, which renders it useless. In the body, the hexamer not only serves as a stable way to store insulin, but it also prevents the monomeric form from aggregating.


Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter