Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Pharmaceuticals
Drug-releasing transplants combat rejection in mice
Researchers attach anti-inflammatory drug to the surface of pancreatic islet transplants using click chemistry
by Tien Nguyen
January 29, 2018

Dressed in shorts and a mustard-colored Hawaiian shirt, Robert R. Kane of Baylor University started his presentation at the inaugural Atlantic Basin Conference on Chemistry in Cancun, Mexico by showing a classic image of human anatomy. Labels on the diagram pointed to major organs that sometimes require replacement.
“Y’all are all familiar with transplantation, and we’re pretty good at it,” he said, speaking on the second day of the conference, which drew 240 attendees from 22 countries, half of whom were students. “But pretty good isn’t good enough.”
Transplant rejection—in which the recipient’s immune system attacks the foreign organ—remains a challenge, he said. Doctors minimize this risk by treating patients with drugs that suppress inflammation and lower their body’s natural defenses. But these treatments can leave patients vulnerable to infections and other side effects.
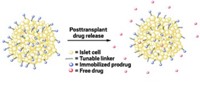
Kane and colleagues have developed a method to attach anti-inflammatory drugs to the surface of transplant tissue in mice (Biomater. 2017, DOI: 10.1016/j.biomaterials.2017.12.020). The new approach localizes the drug to the transplanted organ to thwart acute inflammation and reduce transplant damage and rejection.
The team tested their approach on a well-established transplant model in mice using pancreatic islets with the help of transplant expert Bashoo Naziruddin at Baylor University Medical Center. Pancreatic islets are small cell clusters or “mini-organs” that produce insulin. People with certain types of diabetes, who struggle to manage the disease with medication, sometimes need islet transplants. However, each procedure requires cells from two to three deceased donors because as much as 50–75% of the islets are destroyed by an acute inflammatory response within a couple of days.
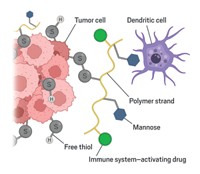
To protect the transplanted islets, the researchers looked to a compound called TAK-242, originally developed to combat sepsis by the company Takeda and known to inhibit a receptor that activates inflammation.
They install the drug on mouse islets in a two-step process. First, a commercially available compound known as NHS-PEG4-DBCO reacts with amine groups on the tissue, anchoring itself to the surface and dangling strained cyclooctyne rings around the tissue edges. The TAK-242 prodrug equipped with an azide linker then “clicks” into the cyclooctynes handles. The linker slowly degrades to release the active drug.
The researchers found that a competing degradation reaction accounts for about 25–30% unproductive loss of the drug. Kane says the team hopes to address this issue by modifying the linker structure.
Diabetic mice receiving the drug-decorated islet transplants experienced no inflammation and after one month, the animal’s glucose levels returned to normal. Treating the islets with free TAK-242 also inhibited inflammation in mice but showed decreased effectiveness by 24 hours after transplantation.
Asier Unciti-Broceta of the University of Edinburgh, who had presented earlier in the session, calls the method very clever and thinks delivering the drug locally should reduce systemic side effects. The key to making this approach viable will be through discussions with clinical practitioners, he says, because surgeons will have to do the chemistry in the operating room so the procedures must be simple.
These are “dump-and-stir” reactions and are stable to air and physiological conditions, Kane says. The team hopes to simplify the chemistry down to a single synthetic step.


Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter