Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Analytical Chemistry
Graphene device reveals step-by-step dynamics of single-molecule reaction
Electrical signals together with theory allow scientists to track reaction on microsecond timescale
by Tien Nguyen
February 14, 2018
| A version of this story appeared in
Volume 96, Issue 8

In introductory organic chemistry courses, students learn about reaction mechanisms, often by drawing neat arrows connecting one intermediate state to the next until ending with a final product. Observing these proceedings in reality, however, is much more difficult.
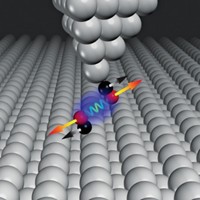
Now, a team of international researchers has made a device that captures the real-time dynamics of a classic chemical reaction at the single molecule level (Sci. Adv. 2018, DOI: 10.1126/sciadv.aar2177). Developed by Xuefeng Guo at Peking University, Kendall N. Houk at UCLA, and Deqing Zhang at the Institute of Chemistry, Chinese Academy of Sciences, the method could illuminate the mechanism of important chemical and biological processes.
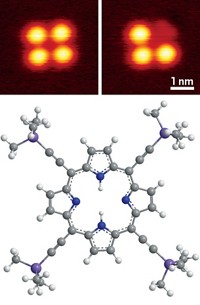
The device consists of two graphene arrays that flank a single molecule covalently secured to each array through amide linkers. The molecule, 9-fluorenone, contains a carbonyl group situated astride three fused rings. The team submerged the device in a solution containing a catalyst and the reagent hydroxylamine, which reacts with 9-fluorenone’s carbonyl group. The reaction changes the electrical charge of 9-fluorenone, so the team could follow the nucleophilic addition reaction by monitoring current conducted by the graphene arrays.
To map the device’s electrical signals to molecular intermediates, the group turned to theory. They calculated rates for each step of the reaction, allowing them to assign lifetimes to reaction intermediates and then match those calculations to experimental data. Using this approach, they identified a reversible transition between the reactant and a new intermediate for the reaction.
Unlike existing single-molecule methods, such as fluorescence microscopy-based ones, this new technique doesn’t rely on adding complex fluorescent labels to the molecule being studied. Also a key advantage of the graphene device is that researchers can monitor reactions with microsecond time resolution, says Peng Chen of Cornell University, who was not involved in the work. “Microsecond time resolution is possible but challenging,” for optical methods, he adds.
“This study presents a powerful and elegant combination of molecular electronics, quantum chemistry and single-molecule chemical physics,” says David Millar of the Scripps Research Institute California. “Chemical reaction kinetics are directly revealed at the single-event level, providing a textbook-like clarity.”


Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter