Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Origins Of Life
How peptides arose from the primordial soup
Amino acid precursors could have reacted to form peptides on prebiotic Earth
by Giuliana Viglione
July 16, 2019
| A version of this story appeared in
Volume 97, Issue 29

Scientists have long wondered how the first complex biomolecules arose from the abiotic ooze. After all, life on Earth today requires enzymes to make basic biomolecules such as peptides—short chains of amino acids—from simple starting materials. Since the 1950s, prebiotic chemists have attempted to understand how these basic components of life could have formed on early Earth, before biological catalysts existed to facilitate these reactions.
Now, University College London (UCL) chemists led by Matthew Powner have come up with a reaction that could have made peptides even before enzymes, and the cells that make them, existed (Nature 2019, DOI: 10.1038/s41586-019-1371-4).
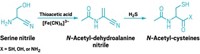
The UCL chemists chose reaction conditions and ingredients thought to be representative of primordial conditions. They used only compounds that could plausibly have existed before the advent of life on Earth, such as hydrogren sulfide, which could have been emitted by volcanoes, and cyanoacetylene, a simple organic molecule that can form from the introduction of electric sparks to a mix of gases. For decades, researchers have hypothesized that these simple molecules could have come together to make biomolecules such as proteins, but just how that would happen on early Earth has not been clear.
Instead of focusing on amino acids as the necessary building blocks, Powner and his team took a step back, focusing on amino acid precursors called aminonitriles, which were also likely around on young Earth. By using the energy inherent in the nitrile bond, they hypothesized that they could make peptides directly from aminonitriles. Direct ligation, or joining together, of aminonitriles is the simplest way that peptides could have formed under prebiotic conditions, but previous work has only been able to achieve this through the use of harsh solvents. Powner wanted to figure out how to make the reaction occur efficiently in water, as it likely would have on young Earth.
The key to the new reaction is the addition of an acyl group to the aminonitrile, turning it into an amidonitrile. The acyl group protects the amine from side reactions that would otherwise prevent the formation of peptides. When an activating agent is added, the amidonitrile readily accepts an aminonitrile monomer, beginning the peptide chain. The researchers demonstrated that the reaction can create peptides from the precursors of all 20 amino acids that life uses to make proteins, and carried it out over a broad range of temperatures and pHs that they believe to be more representative of early Earth than previous work.
Previous attempts at prebiotic peptide synthesis have generally approached the problem using uncontrolled polymerization reactions, requiring a purification step at the end to isolate the product—a step that wouldn’t occur outside a chemistry lab. In contrast, the new method goes nearly to completion, resulting in the high yields reported in the paper.
However, the new method does require the sequential addition of hydrogen sulfide to create an amidothio acid and an oxidant to add each monomer. Despite this, the conditions under which the reaction can be carried out suggests this is the closest we’ve come to understanding the chemistry that occurred at the beginning of life on Earth, says Claudia Bonfio, a postdoctoral fellow at the University of Cambridge who studies prebiotic organic chemistry.
“I think that the results are inspiring,” says Bonfio. “The work is very elegant.” In particular, Bonfio praises the fact that the reaction can be carried out for any proteinogenic amino acid precursor, and works under mild, young Earth–like conditions.
The UCL reaction, she says, “fits perfectly in the prebiotic scenario.”
However, Bonfio is careful to point out that the synthesis is still that of uncoded peptides—the experiments do not yet hint at how peptides began to be encoded by DNA or RNA. She is excited for the field to begin to build on Powner’s work, and sees the combination of peptide synthesis and the formation of genetic material as a natural next step.
So does Powner. His next goal is to create a two-biopolymer system, in which nucleic acids are formed alongside peptides.
“To apply this in the origins of life,” Powner says, “we don’t just want to make the peptides. We want to make all the other components that are important in the cell.”


Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter