Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Biochemistry
Taking a wider look at tau modifications
Semisynthesis aids study of posttranslational modifications to key Alzheimer’s protein
by Stu Borman
May 14, 2018
| A version of this story appeared in
Volume 96, Issue 20

In the body, tau protein regulates nerve cell growth, nerve signal transmission, and the function of cytoskeletal components called microtubules. But it can also misfold, ending up in aggregates called neurofibrillary tangles in the brains of Alzheimer’s disease patients, making tau a diagnostic and therapeutic target.
Cells modify tau extensively by adding a range of extra chemical groups, called posttranslational modifications (PTMs), to the protein. But studies of these modifications up to now have focused primarily on one type of PTM—phosphorylation—in one part of tau—its C-terminal region. So tau’s PTM code, how the modifications affect tau’s behavior and aggregation potential, is mostly unknown.
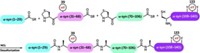
After three years of effort, Hilal A. Lashuel and Mahmood Haj-Yahya at the Swiss Federal Institute of Technology, Lausanne, have developed a tau semisynthesis that could open a wider window on the protein’s PTM code (J. Am. Chem. Soc. 2018, DOI: 10.1021/jacs.8b02668).
They synthesized four fragments that make up the protein’s C-terminal and microtubule-binding regions, areas with tau’s most significant PTMs. They then used ligation reactions to combine these synthetic segments and a bacterially expressed N-terminal fragment to yield full-length tau with a completely native sequence.
The researchers can modify each synthetic domain with one or more PTMs before assembling the full protein. Using this method, they showed that acetylation at lysine 280 in the microtubule-binding domain enhances tau aggregation, implicating that PTM as a possible drug target.
“It’s a tour de force of semisynthesis and clearly an advance in the field of tau research,” says tau PTM specialist Christian P. R. Hackenberger of Humboldt University of Berlin.


Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter