Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Cancer
Microtubule protein level predicts drugs’ effectiveness against brain cancer cells
Lab study shows that drugs like paclitaxel are more effective against glioblastoma cells with higher levels of tubulin
by Cici Zhang, special to C&EN
September 10, 2019

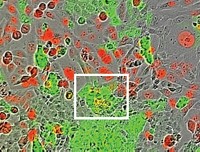
Certain chemotherapy drugs, including paclitaxel (Taxol), block cancer cell division by targeting microtubules—structural polymers that are critical for cell division and other functions. Such microtubule-targeting agents (MTAs) are promising drug candidates against glioblastoma, an aggressive brain cancer, but they have not reached the clinic in part because of highly variable results in the lab. Now, researchers have found one reason why MTAs do not stop the growth of all glioblastoma cells: different levels of microtubule proteins in these cells affects their susceptibility to MTAs (ACS Pharmacol. Transl. Sci. 2019, DOI: 10.1021/acsptsci.9b00045).
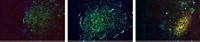
Microtubules contain polymerized tubulin dimers made of an α-tubulin and a β-tubulin, each of which comes in several forms. Although researchers know that MTAs bind to tubulins to disrupt microtubule function, not much is known about how a cell’s tubulin profile might impact the drugs’ effectiveness. So Lenka Munoz of the University of Sydney and her team analyzed 15 glioblastoma cell lines and characterized the amounts of tubulin proteins using immunoassays. Then they tested four small MTAs that can potentially cross the blood-brain barrier—colchicine, nocodazole, tivantinib, and CMPD1—against the cell lines. In all cases, cells with higher α- and β-tubulin levels were more susceptible to MTAs—including the 12 lines of stem-cell-like glioblastoma cells that the team tested. In the battle against the notoriously aggressive cancer, these cells are of particular interest because they can escape chemotherapy and cause a recurrence of the cancer.
To validate their finding, the researchers also tested three MTAs commonly used in the clinic: paclitaxel, vinblastine, and ixabepilone. In four key stem-cell-like cell lines that survived these drugs, tubulin levels were as much as 20% lower than lines that were effectively killed by MTAs, the researchers found. Why cells have different levels of tubulin is not known, Munoz says.
In addition, the researchers observed that cells with low tubulin levels and high MTA tolerance had more physical and molecular features typically found in nonproliferating, dormant cancer cells. This suggests to the researchers that low-tubulin-containing cells somehow convert to a dormant state during which they do not divide. That way, the cells survive MTA treatment and can proliferate once the MTA therapy is finished.
The team is working to understand the molecular mechanisms behind this switch to dormancy, information that researchers could use to design drugs to prevent cancer cells from going into that state, Munoz says. She says physicians could then use such drugs along with MTAs to eradicate all cells within a brain tumor.
Maria G. Castro, a brain cancer scientist at the University of Michigan Medical School who does both basic and translational research, calls the data from this study “exciting” and says that this work paves the way for personalized therapy when using MTAs to treat brain cancer. The next critical steps would be to test the findings in animal models using cells with different MTA sensitivity, she says.


Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter