Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Gene Editing
Base editing targets progeria mutation in mice
Findings are the first step toward a treatment for accelerated aging disease
by Celia Henry Arnaud
January 6, 2021
| A version of this story appeared in
Volume 99, Issue 2

The gene-editing method known as base editing might be a way to address the underlying cause of Hutchinson-Gilford progeria syndrome (HGPS or progeria), a rare genetic disease that leads to accelerated aging. Children with this mutation develop severe cardiovascular problems and typically survive only until their early teens. Correcting the mutation using the base-editing approach extends the life of mice with a version of the human disease, according to a new study (Nature 2021, DOI: 10.1038/s41586-020-03086-7). The approach could be useful for treating other rare diseases caused by point mutations, the researchers say.
In 2003, researchers in Francis S. Collins’s lab at the US National Institutes of Health identified a point mutation in the gene for a protein known as lamin A as the most common cause of progeria. The mutation, in which a T-A base pair replaces a C-G base pair, leads to incorrect RNA splicing and results in a version of lamin A, known as progerin, with an internal deletion.
As a result of the deletion, progerin permanently carries a farnesyl group at one end. Farnesyl groups help proteins associate with lipid membranes, but the groups are eventually cleaved off in healthy cells. Progerin ends up damaging the cell’s nucleus, especially in the heart and blood vessels. In November, the US Food and Drug Administration approved the first drug for progeria—lonafarnib, which is being marketed by Eiger BioPharmaceuticals under the trade name Zokinvy—a small molecule that inhibits the farnesylation process.
Collins, who was involved in research on lonafarnib, celebrates the accomplishment. “But if we really want to believe that science can come to the rescue here, we had to keep going,” Collins says. He wanted to address the underlying mutation behind progeria. Traditional gene therapy, which supplies a healthy copy of a gene to replace something lacking, cannot help in this case; a single copy of the mutated gene causes the disease, so the damaging mutation must be fixed.
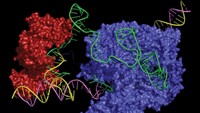
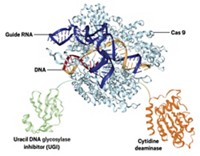
Meanwhile, David R. Liu of Harvard University and Jonathan D. Brown of the Vanderbilt University Medical Center had already used adenine base editing to target the mutation that causes progeria in a preliminary study. Adenine base editors, developed in Liu’s lab in 2017, are engineered enzymatic machines derived from the CRISPR-Cas9 gene-editing system that target a specific gene sequence and convert a single adenosine to inosine, a nucleoside that can take the place of the standard guanosine. The cell’s normal DNA repair machinery then takes over and replaces the thymine with cytosine in the unedited strand, using the inosine as a template. This swap is carried through subsequent cell replication cycles.
In the new study, Collins, Liu, and Brown collaborated to deliver a base editor designed to correct the progerin mutation. The base editing worked in cultured cells derived from children with progeria and in mice engineered to carry copies of the mutated human lamin A gene. Mice that received the base editor at 14 days old lived more than twice as long as mice that didn’t receive the treatment.
“To date this is the closest approach to a cure for HGPS as it has the potential for not only extending the lifespan of HGPS patients but also significantly improving quality of life,” Robert D. Goldman and Stephen A. Adam, experts on lamin protein diseases at Northwestern University, write in an email.
“The greatest challenges will be working out the dosage and timing in human patients,” Goldman and Adam continue, especially since there are so few people with progeria. Such base editing has not been done in humans before.
Maria Eriksson of the Karolinska Institute, a progeria expert who was the lead author on the paper from Collins’s lab that originally identified the mutation, agrees that it remains to be seen whether base editing will work with more fully progressed disease. In this study, the mice had not showed signs of progeria when the mutation was corrected, she says in an email, but in children, the disease is typically identified later.
The researchers have begun discussions with Beam Therapeutics, a company Liu founded, to develop a potential progeria treatment. “This is one of the most absolutely satisfying experiences I’ve had to see how you can bring really exciting new technology to bear on a problem that desperately needs a new solution,” Collins says. “We’re not there yet,” he adds, “but I’m excited to see how we might get there.”


Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter