Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Infectious disease
Covid-19
Emory-discovered antiviral is poised for COVID-19 clinical trials
The nucleoside inhibitor has advantages over Gilead’s remdesivir but has yet to be tested in humans
by Lisa M. Jarvis
March 26, 2020
| A version of this story appeared in
Volume 98, Issue 12

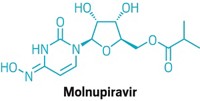
A small-molecule antiviral discovered by Emory University chemists could soon start human testing against COVID-19, the respiratory disease caused by the novel coronavirus. That’s the plan of Ridgeback Biotherapeutics, which licensed the compound, EIDD-2801, from an Emory nonprofit.
EIDD-2801 works similarly to Gilead Sciences’ remdesivir, an unapproved drug that was developed for the Ebola virus and is being studied in five Phase III trials against COVID-19. Both molecules are nucleoside analogs that metabolize into an active form that blocks RNA polymerase, an essential component of viral replication.
But remdesivir can only be given intravenously, meaning it would be difficult to deploy widely. In contrast, EIDD-2801 can be taken in pill form, says Mark Denison, a coronavirus expert and director of the infectious diseases division at Vanderbilt Medical School. Denison partnered with Emory and researchers at the University of North Carolina to test the compound against coronaviruses.
Support nonprofit science journalism
C&EN has made this story and all of its coverage of the coronavirus epidemic freely available during the outbreak to keep the public informed. To support us:
Donate Join Subscribe
EIDD-2801 has other promising features. Many antivirals work by introducing errors into the viral genome, but, unlike other viruses, coronaviruses can fix some mistakes. In lab experiments, EIDD-2801 “was able to overcome the coronavirus proofreading function,” Denison says.
He also notes that while remdesivir and EIDD-2801 both block RNA polymerase, they appear to do it in different ways, meaning they could be complementary.
Unlike remdesivir, EIDD-2801 lacks human safety data. Ridgeback founder and CEO Wendy Holman says she expects the US Food and Drug Administration to give the green light for a Phase I study in COVID-19 infections within “weeks, not months.”



Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter