Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
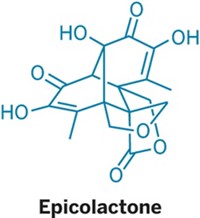
Natural Products
How to make canataxpropellane in 26 steps
First synthesis of complex taxane creates sufficient quantities to study its biological properties
by Bethany Halford
February 6, 2020
| A version of this story appeared in
Volume 98, Issue 6
Meet the popular anticancer drug Taxol’s quirky cousin: canataxpropellane. Chemists at the University of Konstanz have now synthesized the elusive compound for the first time, creating enough of the taxane for scientists to study its bioactivity (Science 2020, DOI: 10.1126/science.aay9173).
Because canataxpropellane’s natural source, the Taxus canadensis shrub, ekes out the compound in minuscule quantities, scientists haven’t been able to evaluate its biological properties. Even though it has just 24 carbon atoms, canataxpropellane’s complex architecture makes it a beast to synthesize. It has Taxol’s core skeleton, but it also contains three additional C–C bonds that bridge the compound. Those three bonds “totally change the overall strategy of approaching the molecule” so that it must be distinct from Taxol syntheses, says Tanja Gaich, who led the synthetic effort.
Canataxpropellane’s other structural challenges include two propellanes—motifs in which three rings share a single C–C bond. Canataxpropellane has 12 contiguous stereocenters. Five of those are tough-to-make quaternary centers, where a carbon atom bonds to four other carbons. And four of those reside inside a cyclobutane ring.
The key strategic step comes early in the synthesis, when the chemists assemble the cyclobutane ring and four of its quaternary chiral centers using an alkene-arene-ortho photocycloaddition. Gaich says she wasn’t surprised that this strategic step worked but was pleased that it worked as well as it did, enabling the chemists to make the intermediate on a multigram scale. The next step in the synthesis, which should have been a simple lactol opening, failed all attempts. It took the chemists nearly a year and a half to figure out a work-around. In all, the synthetic project took nearly 5 years.
Gaich’s group constructed the molecule in 26 steps from a known intermediate; it would take 29 steps to make it from commercially available material. The overall yield was 0.5%, with many of the steps done on gram or multigram scale.“This is a highly creative strategy to construct a dauntingly complex molecule,” says Richmond Sarpong, a chemist at the University of California, Berkeley. The team’s synthetic approach, he says, is “a beautiful solution.”


Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter