Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Synthetic Biology
Reaction cascade modifies proteins
2-step, 1-pot method uses protein splicing to add chemical moieties to proteins
by Celia Arnaud
July 5, 2019
| A version of this story appeared in
Volume 97, Issue 27

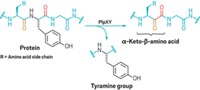
Chemically modifying proteins with imaging probes or nonnatural amino acids can be a powerful way to study biological processes. But existing methods for introducing modifications work only at high reactant concentrations and can leave unwanted amino acid “scars.” A new method developed by Tom W. Muir and coworkers at Princeton University overcomes these limitations (Nat. Chem. 2019, DOI: 10.1038/s41557-019-0281-2). Their method uses self-splicing protein segments called inteins. They made the inteins in two parts, one of which is decorated with the moeity the researchers want to add to the protein, the other of which is attached to the protein. The first half is split into two pieces, including a short “overhang” of 10 or fewer amino acids. Step 1 of the process (shown) involves reconnecting the overhang, which carries the desired modification, to the rest of the truncated intein via an enzymatic transamidation reaction. Then in step 2 (shown), the truncated intein reacts with its other half. After the two halves are reconnected, they splice themselves out, leaving the chemical modification and no more than an extra cysteine on the recombinant protein. The whole process occurs as a single-pot reaction cascade at low peptide and protein concentrations. The researchers were able to add multiple chemical modifications to a single protein. The method works with isolated proteins and with more complex structures such as cellular chromatin.


Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter