Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Start-ups
Epitranscriptomics: The new RNA code and the race to drug it
A small group of scientists studying chemical modifications on RNA ushered in the field of epitranscriptomics. Now they’re hoping it will create an entirely new way to treat cancer
by Ryan Cross
February 18, 2019
| A version of this story appeared in
Volume 97, Issue 7

Credit: Storm Therapeutics | Methyl groups (represented by the glowing circle) added to RNA (long strand), are part of the epitranscriptomic code. Biotech start-ups are now trying to drug the proteins that add these methyl groups to RNA.
In brief
For decades, scientists knew that RNA was decorated with chemical modifications. But without any good way to measure or map these modifications, most researchers either forgot about them or just thought they were boring. Now, the creation of tools to study modified RNAs has ushered in a new field called epitranscriptomics. Classes of proteins called writers, erasers, and readers that make, remove, or recognize these modifications are increasingly being linked to cancer. A trio of biotech start-ups, founded by leaders in the field, is hoping to establish a new class of cancer medicines that target these editors of RNA.
It’s not every day that a biotech investor stumbles across an entirely new field of science. And frankly, Carlo Rizzuto wasn’t even looking for such a thing. When Rizzuto, a partner at the venture capital firm Versant Ventures, embarked on a scouting trip to New York City in 2014, he was simply hoping to discover academic research that was ripe enough to form the basis of a biotech company.
Rizzuto had an appointment with Samie Jaffrey, an RNA scientist at Weill Cornell Medicine. RNA is often described as a cousin to DNA—the stuff that our genes are made of. One kind of RNA, called messenger RNA, acts as the intermediary code that cells use to transfer information stored in DNA into a set of instructions that cells can easily read for making proteins.
After Rizzuto rejected several of his projects, Jaffrey mentioned a relatively young line of work focused on studying chemical modifications to RNA. In 2012, his lab invented a method to map the location of methyl groups that, for some reason, cells were adding to their mRNA. It was reminiscent of another field, called epigenetics, or the study of chemical modifications made to DNA to turn genes on or off. The entirety of RNA in a cell is called the transcriptome, so Jaffrey dubbed the new field “epitranscriptomics.”
Rizzuto perked up. “This is something that we would be very interested in,” he said.

Jaffrey was hesitant. “We’re just doing basic stuff now,” he recalls explaining. His lab, and others, was still trying to figure out how this RNA modification system worked. They were building evidence suggesting that enzymes added and removed these methyl marks to control the fate of mRNA, and thus protein production, but many questions remained. Jaffrey implored: “Carlo, what disease would we be curing if we started a company around epitranscriptomics?”
“It doesn’t matter,” Rizzuto replied. “This is so central to molecular biology; it has to be related to fundamental disease processes.”
Then reality kicked in. Venture capital firms like Rizzuto’s aren’t in the business of funding years of basic research just to see if something like epitranscriptomics is involved in disease. “We were looking at a new paradigm for gene-expression regulation,” Rizzuto recalls, but it was too early to start a company. He and Jaffrey agreed to stay in touch.
Rizzuto’s enthusiasm in 2014 has since percolated among scientists and investors learning about epitranscriptomics. Several groups, including Jaffrey’s, have shown that the epitranscriptomic code—the number and location of chemical modifications across a cell’s RNA—is seriously out of whack in some cancers. And with basic tools in hand to read this previously hidden layer of information in cells, biotech companies are now out to alter it. Three start-ups, including one that Jaffrey and Rizzuto helped found, called Gotham Therapeutics, have launched with more than $110 million in total dedicated to epitranscriptomics drug discovery.
There was a similar reaction to epigenetics more than a decade ago, when it became clear that chemical modifications regulating genes are frequently out of whack in cancer. Companies rushed to develop drugs against proteins responsible for making, removing, and recognizing chemical modifications on genes—often referred to as the writer, eraser, and reader proteins. With the discovery of parallel writer, eraser, and reader proteins working on RNA, epitranscriptomics is looking like a promising, untapped area for drug discovery.
But there’s another parallel to epigenetics that’s less optimistic: thus far, epigenetic drugs have been a disappointment. “Epigenetics turned out to be a lot more complicated than the community originally thought,” says Chuan He, a professor of chemistry at the University of Chicago.
He, a scientific founder of the epitranscriptomics company Accent Therapeutics, has been at the forefront of developing the new study of RNA modifications and their role in disease. He, Jaffrey, and many others are confident that understanding and controlling RNA modifications will provide completely new avenues for treating disease. “What this really offers is a totally new biology,” He says. “And whenever there is a new biology emerging there are always opportunities for therapies.”
Epitranscriptomics, abridged
A series of discoveries and technical advancements over the past decade has spawned a new field called epitranscriptomics, the study of chemical modifications to RNA, and the proteins that write, erase, and read these modifications. In recent years, studies implicating epitranscriptomic proteins in cancer have led to the launch of three biotech companies dedicated to drugging these proteins.
May 2008: Rupert Fray shows that a methyl-adding enzyme is essential for plant development. The study inspires others to look at RNA modifications.
November 2010: Chuan He proposes new field of RNA epigenetics, suggesting that methyl modifications on RNA can be removed.
October 2011: Chuan He’s lab proves that an enzyme called FTO erases methyl modifications on RNA.
April and May 2012: The labs of Gideon Rechavi (April) and Samie Jaffrey (May) publish the first maps of RNA methyl modifications. Jaffrey coins the word “epitranscriptomics.”
October 2014: Howard Chang’s lab shows that METTL3, which adds methyl groups to RNA, is critical for embryonic stem cell development and differentiation.
June 2016: Storm Therapeutics, founded by University of Cambridge scientists Tony Kouzarides and Eric Miska, raises $16 million to drug proteins that make RNA modifications.
September and November 2017: Independent studies from Samie Jaffrey and colleagues (September) and Tony Kouzarides and colleagues (November) show that METTL3 is elevated in acute myeloid leukemia and that suppressing the enzyme forces the cancer cells to become noncancerous.
May 2018: Accent Therapeutics, cofounded by Chuan He, Howard Chang, and Robert Copeland, raises $40 million.
October 2018: Gotham Therapeutics, cofounded by Samie Jaffrey, launches with $54 million.
February 2019: Evidence builds that epitranscriptomics may be important for cancer immunotherapy. Chuan He shows that deleting a reader protein boosts the efficacy of checkpoint inhibitors in mice.
Making a map
A series of events beginning in 2008 laid the foundation for epitranscriptomics. That year, while He was studying epigenetic enzymes that remove methyl modifications from DNA, he and University of Chicago biologist Tao Pan began doubting that all these enzymes were really working on DNA as others assumed. The evidence was particularly shaky for one enzyme, called fat mass and obesity-associated protein, or FTO.
He and Pan wondered if FTO removed methyl groups from RNA instead—an idea that might sound simple enough today but was unorthodox at the time. Scientists had discovered methyl modifications on RNA decades earlier, yet with no good way to measure or map these modifications, most people forgot about them.

But a study coming out of the lab of plant biologist Rupert Fray at the University of Nottingham reinforced He and Pan’s suspicions that RNA modifications were underappreciated. Fray showed that plants missing a methyl-adding enzyme—similar to an enzyme called METTL3 in humans—stopped growing at a specific early stage in their development.
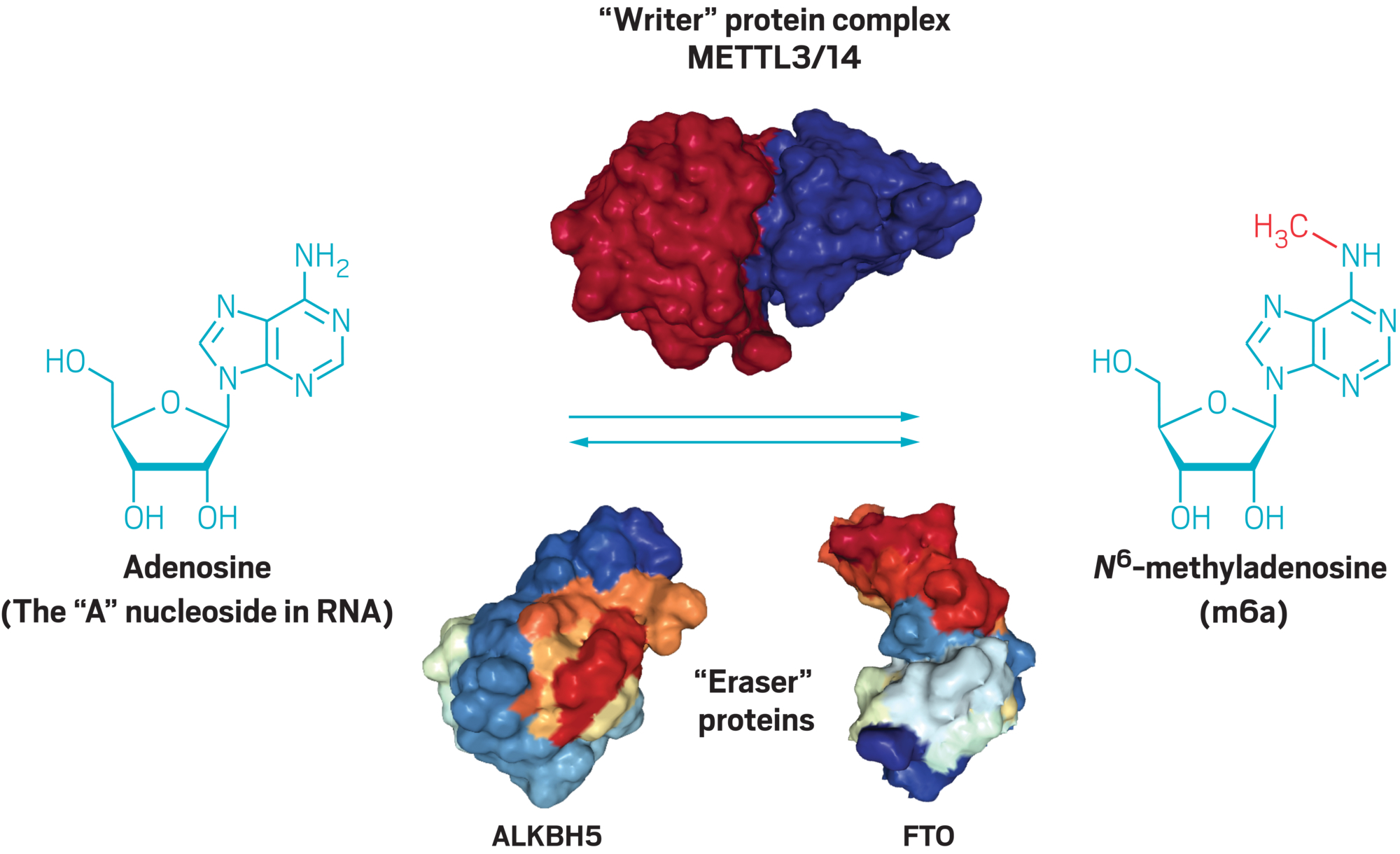
Scientists knew that METTL3 placed a methyl on a specific nitrogen in adenosine, one of the four building blocks of RNA. This modified building block is called N6-methyladenosine, or m6A for short. Beyond m6A, chemists had cataloged some 150 different chemical modifications to RNA in bacteria, plants, and animals. If He could find an enzyme that removed the methyl groups, it would suggest that there was an undiscovered RNA control system in cells, analogous to epigenetic controls in DNA.
In 2010, He coined the phrase “RNA epigenetics” in a commentary that outlined his ideas (Nat. Chem. Biol., DOI: 10.1038/nchembio.482). A year later, He and Pan published evidence showing that the FTO enzyme was an eraser—it removed the methyl modifications made by METTL3 (Nat. Chem. Biol. 2011, DOI: 10.1038/nchembio.687).
METTL3 and FTO are both enzymes, which means they should be pretty straightforward to inhibit with small-molecule drugs. That notion would later be frequently cited by the new epitranscriptomics companies, although it would be several years still before these enzymes were connected to disease.
At first, the significance of these enzymes was lost on many researchers. At Weill Cornell, however, Jaffrey immediately recognized that He’s study was part of a new field that was about to explode. His lab had been working on a method to detect and map m6A across a cell’s mRNA. Jaffrey had also seen Fray’s work on m6A in plants and thought that if the modifications existed in humans, they must be doing something important in us too.
At the time, methods for studying m6A were rudimentary. Researchers could detect the presence of m6A in ground-up globs of mRNA run through common chemistry lab techniques like chromatography or mass spectrometry. “But you had no idea which mRNAs were being modified,” Jaffrey says. No one knew if all mRNA had some m6A or if the methyl modifications were found on only certain transcripts, he adds. “And frankly, it wasn’t even terribly clear that m6A levels changed.”
So Jaffrey and Kate Meyer, a postdoc in his lab, developed a technique to figure out which mRNAs contained these modifications. They used commercially available antibodies that attach to m6A to fish out fragments of human mRNA for sequencing (Cell 2012, DOI: 10.1016/j.cell.2012.05.003).
That technique allowed the creation of the first map of m6A. The results were stunning. “We thought that m6A was going to be all over the place, kind of random,” Jaffrey says. Instead, the researchers saw that methyl marks tended to cluster near an area called the stop codon, and only on certain mRNA transcripts. “It was so specific, it just knocked our socks off.”
Advertisement
An even closer inspection revealed that many of the mRNAs containing m6A were linked to differentiation and development, the same functions that were affected in Fray’s stunted plant embryos. “We were amazed,” Jaffrey says.
In April 2012, while Jaffrey and Meyer were waiting for their m6A paper to publish, another group, led by Gideon Rechavi at Tel Aviv University, published its own paper on the use of antibodies to map m6A in mouse and human cells (Nature 2012, DOI: 10.1038/nature11112). “It was met with a lot of skepticism,” says Dan Dominissini, the PhD student in Rechavi’s lab who led the project. “People didn’t get why it was important. It took a year to publish.”
The problem was researchers still hadn’t established a clear link between these RNA modifications and disease, or even basic human biology. Moreover, the field wouldn’t have its name of epitranscriptomics for another three weeks, when Jaffrey and Meyer’s paper describing their m6A-mapping technique was published online in May 2012. Although Jaffrey had been scooped, the back-to-back publications put epitranscriptomics on the radar. The field was poised to explode.
Editing the epitranscriptomic code
Searching for disease
In Chicago, He was positioning his lab as the forefront of epitranscriptomics research. His group discovered that an enzyme called ALKBH5, like FTO, erased methyl marks on RNA, turning m6A back into adenosine. Yet even by 2014, two years after the m6A-mapping methods were published, epitranscriptomics wasn’t getting the recognition, or funding, that He thought it deserved. “People thought it was cute,” He says. “But biologists were not convinced of its significance.”
Work by Stanford University geneticist Howard Chang helped change that attitude. Chang showed that without METTL3, mouse and human embryonic stem cells grow uncontrollably and never undergo their normal maturation process, called differentiation, in which they change into specialized cells, such as muscles, neurons, or white blood cells (Cell Stem Cell 2014, DOI: 10.1016/j.stem.2014.09.019). A few months later, Rechavi’s group published a similar study, with an added step showing that mouse embryos missing METTL3 died before birth.

It was becoming clear that m6A was not just some random mark on a transcript. Even though the methyl group is physically small, it has a big effect on RNA. The modifications can coax RNA to assume a different 3-D structure. He’s lab also began studying newly discovered proteins dubbed readers, which have small pockets that bind m6A. He showed that one reader specifically binds m6A to flag an mRNA for destruction. Another reader binds m6A to help spur protein production. He’s elucidation of these reader proteins increased interest in epitranscriptomics for scientists and investors, says Larry Lasky, a partner at the Column Group, a venture capital firm. “It started to look and smell like epigenetics.”

Epitranscriptomics was now a hot topic. As studies began bubbling up exploring the role of RNA modifications, particularly m6A, in a variety of cells and species, investors started putting money into the field. In June 2016, a British start-up called Storm Therapeutics raised $16 million and became the first company dedicated to tackling the new RNA epigenetics.
Although Storm was several years in the making, it wasn’t clear what diseases the company would be curing. Two University of Cambridge scientists, Tony Kouzarides and Eric Miska, began discussing the idea for the company back in 2012, when they had published work on obscure enzymes that chemically modify microRNAs, which regulate the function of other RNAs.
Although the enzymes were linked to cancer, at least in cells growing in a dish, the microRNA studies went largely unnoticed. Kouzarides and Miska thought more undiscovered links between RNA modifications and cancer must exist, but it took a few years to find investors willing to bet on their hypothesis. “I don’t think that there was a huge amount of actual data; it was just the belief that there must be,” Storm’s CEO, Keith Blundy, says. “The idea that all of these chemical modifications on RNA weren’t dysregulated or mutated or changed in cancer was almost unthinkable.”
That belief, which echoes the sentiment that Versant Ventures’ Rizzuto expressed in Jaffrey’s office in 2014, was about to be validated. In the second half of 2016, studies began linking reader and writer proteins to cancer. Jaffrey saw the evidence firsthand in an ongoing study he was conducting in blood cancer. The implications for drug discovery were becoming clear. He reached out to Rizzuto. It was time to move forward.
Annotations in the blood
The common thread running through epitranscriptomics research was its link to cell differentiation and development. Chang’s and Rechavi’s stem cell studies on m6A gave several research labs—including He’s, Jaffrey’s, and Kouzarides’s—the idea to look at the role of these RNA modifications in a deadly blood cancer called acute myeloid leukemia.
Leukemia is essentially a disease of dysfunctional differentiation. Healthy people’s bones are filled with hematopoietic stem cells that produce white blood cells. In leukemia, these stem cells go haywire. They proliferate and displace other blood cells because they can’t differentiate, or mature, into normal white blood cells.
In December 2016, He’s lab, together with several collaborators, showed that tissue samples taken from people with certain kinds of acute myeloid leukemia displayed high levels of the enzyme FTO—which, five years earlier, He had discovered is an m6A eraser (Cancer Cell 2016, DOI: 10.1016/j.ccell.2016.11.017). A few months later, with a different set of collaborators, He showed that levels of the methyl-removing enzyme ALKBH5 were elevated in glioblastoma stem cells (Cancer Cell 2017, DOI: 10.1016/j.ccell.2017.02.013).

At the beginning of 2017, Lasky, the Column Group investor, reached out to He. Now that epitranscriptomic enzymes were tied to cancer, Lasky’s firm wanted to start a drug company to control RNA modifications. With the new cancer data in hand, He felt that the time was right.
The investors also knew about a publication in the works from Jaffrey and leukemia expert Michael Kharas at Memorial Sloan Kettering Cancer Center. The Column Group and Versant Ventures worked together for a time to begin forming a single epitranscriptomics company with several of the academic leaders. During the summer of 2017 however, the different players split into two camps. The Column Group brought on He and Chang as academic cofounders of Accent Therapeutics. Versant Ventures named Jaffrey the academic founder of Gotham Therapeutics.
While Accent and Gotham were still in stealth mode, Jaffrey published a study showing that genetic mutations led to fixed, elevated levels of METTL3 in acute myeloid leukemia, keeping white blood cells from forming. By reducing METTL3 levels, leukemia cells could be coaxed into undergoing differentiation to become noncancerous cells that eventually die (Nat. Med. 2017, DOI: 10.1038/nm.4416). “It was remarkable because we didn’t even need complete inhibition of METTL3,” Jaffrey says.
Two months later, Kouzarides’s lab at the University of Cambridge published similar results, with additional details on what METTL3 was doing in these cells (Nature 2017, DOI: 10.1038/nature24678). In leukemia, elevated METTL3 encouraged the production of proteins linked to cancer. “It is feeding the cell the very proteins that are driving tumorigenesis,” Gotham CEO Lee Babiss says.
Epitranscriptomics now had drug targets, diseases, and high-profile studies. After recruiting additional investors, Accent launched with $40 million in May 2018, and Gotham launched with $54 million in October. Storm Therapeutics is in the process of raising approximately $65 million for its second round of cash from investors. Although none of these companies will name their targets or first diseases they will attempt to treat, conversations with the companies’ CEOs suggest that developing inhibitors of METTL3 is a goal for all three.
Drug designers have a lot of experience inhibiting enzymes, making METTL3 an attractive first target. But its activity may not be straightforward, says Yunsun Nam, a biophysicist at the University of Texas Southwestern Medical Center. METTL3 grabs the methyl group it adds to RNA from S-adenosylmethionine (SAM), a molecule used by several other enzymes. Companies’ compounds will need to avoid inhibiting these other enzymes as well, she explains.
Nam thinks a workaround could be targeting a protein called METTL14, which is attached to METTL3 as part of a larger m6A-writing complex. “METTL3 and METTL14 are very dependent on each other for stability,” she says.
Even if the companies can develop selective METTL3 inhibitors, it’s unclear how many people would benefit from them. While the leukemia studies by Jaffrey and Kouzarides showed that m6A levels are too high, He’s leukemia and glioblastoma studies showed the opposite, that m6A levels are too low. Other studies have suggested more contradictory results—including that m6A levels may be too high in glioblastoma. In other words, when developing therapies, it will be crucial to know the epitranscriptomic state of one’s cancer cells. Otherwise, giving the wrong person a METTL3 inhibitor might make things worse.
“That’s a possibility,” Robert Copeland, the president and chief scientific officer of Accent, acknowledges. The challenge for Accent and other companies will be to figure out which subset of people with leukemia would benefit from a METTL3 inhibitor, to lower m6A levels, and which would benefit from an FTO inhibitor, to raise m6A levels, Copeland explains. “If the pendulum swings too much one way or too much the other way, you can cause disease.”

Epi-expansion
Although the leaders of Accent, Gotham, and Storm are being secretive about their strategies, they all hint that the potential scope of epitranscriptomics drug discovery is much bigger than just targeting METTL3.
In addition to the m6A erasers, a growing body of work is uncovering the importance of the m6A readers. Earlier this month, He’s lab showed that an m6A reader protein called YTHDF1 is an important control switch in the immune system and that inhibiting it might dramatically boost the efficacy of existing checkpoint inhibitors, a popular class of cancer immunotherapy (Nature 2019, DOI: 10.1038/s41586-019-0916-x). “I think a lot of immunotherapy companies will jump into epitranscriptomics once they read the paper,” He says.
And this isn’t the first known link between epitranscriptomics and immunotherapy, Accent’s Copeland says. His firm has been studying an enzyme called ADAR1—which stands for adenosine deaminase acting on RNA—that modifies adenosine bases in RNA. Studies from academic labs show that some tumors depend on ADAR1 in ways that normal cells do not. One study suggests that blocking ADAR1 could make certain drug-resistant cancers vulnerable to checkpoint inhibitors (Nature 2018, DOI: 10.1038/s41586-018-0768-9).
Other labs entering the fray are uncovering new proteins that read, write, and erase RNA modifications, with links to additional types of cancer and other diseases. The scope of epitranscriptomics could be enormous. “That’s what excites us about the field,” says Blundy, Storm’s CEO. “There are many, many RNA pathways that are regulated through modifications.”
The discoveries aren’t all coming smoothly, however. For example, Jaffrey claims that the main target of the eraser enzyme FTO isn’t actually m6A but a slightly different modification, called m6Am. Others disagree. “There is still some debate, but that is the normal trajectory for a field, especially in the early days,” Jaffrey says.
The field also still has technical hurdles. “Right now the methods to map and detect m6A are crude,” Jaffrey admits. Existing methods require large sample sizes and are ineffective at quantifying how m6A levels change over time on particular mRNA transcripts. His lab is now working on ways to better quantify m6A to diagnose or predict diseases in the clinic. Such tools will be critical for recruiting the right people into clinical studies testing inhibitors of epitranscriptomic proteins.
Another issue is the lack of publicly available small-molecule inhibitors for studying epitranscriptomic proteins. “We don’t even have an inhibitor for research,” says Dominissini, who has also developed new RNA-modification-mapping techniques and now runs his own epitranscriptomics lab at Tel Aviv University. Right now, researchers have to use genetic techniques to remove or block production of writer, eraser, and reader proteins, but what the field really needs are simple small molecules to test the hypotheses that these proteins will make good drug targets, he says. Of course, that’s what the companies are working on.
A similar lack of compounds stalled epigenetics drug discovery more than a decade ago. Pioneers in the epitranscriptomics field are unfazed by these parallels. “I don’t think there is a relationship between the success or failure of an epigenetics drug to an epitranscriptomics drug,” Jaffrey says.
The scientists and companies in the field are running full speed ahead. Hundreds of labs have cited papers from Dominissini, He, and Jaffrey, and all can point to several ongoing studies investigating the role of RNA modifications in other diseases. “It reflects how fast people jumped into the field,” He says. “Epitranscriptomics is booming.”




Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter