Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Start-ups
Vicinitas Therapeutics launches with $65 million for protein stabilization
The spin-out from UC Berkeley and Novartis saves needed proteins from destruction
by Gina Vitale
July 28, 2022

Vicinitas Therapeutics has launched with $65 million in series A funding with the goal of saving proteins that are erroneously tagged for disposal. This protein stabilization platform, which was developed through a collaboration between researchers at the University of California, Berkeley, and the Novartis Institutes for Biomedical Research, will be geared to developing therapeutics for cancer and genetic disorders, the company says.
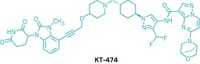
The molecules behind Vicinitas consist of two distinct ends connected by a linker. One end recruits a protein of interest that has been marked with ubiquitin, indicating to the body that it can be destroyed. The other end recruits an enzyme called deubiquitinase, which removes the ubiquitin tags, effectively un-labeling the protein as trash. The double-sided compounds are called deubiquitinase-targeting chimeras, or DUBTACs.
“I think our DUBTAC and targeted protein stabilization approaches really for the first time allow us to stabilize these aberrantly degraded proteins to treat disease,” says Daniel Nomura, Vicinitas’s founder and a chemical biologist at UC Berkeley.
In a recent paper, researchers in Nomura’s lab and at Novartis showed that a DUBTAC could effectively stabilize levels of a protein whose absence is implicated in cystic fibrosis, an often fatal disease caused by a genetic mutation. They also showed that a DUBTAC could stabilize an enzyme that suppresses tumor growth (Nat. Chem. Biol. 2022, DOI: 10.1038/s41589-022-00971-2).
In concept, DUBTACs are the exact opposite of their more common counterparts, proteolysis-targeting chimeras (PROTACs). PROTACs also have two ends; one binds to a disease-causing protein of interest, and the other end to an enzyme that marks the target protein for disposal with ubiquitin.
There have been many advances in the PROTAC field, says Nomura, who also cofounded protein degradation firm Frontier Medicines. “But for the aberrantly degraded targets that you want to stabilize, there hasn’t been a parallel technology to stabilize those until we have developed this DUBTAC platform, which now enables us to access these large swaths of previously intractable disease targets,” he says.
Several challenges make clinical development of both PROTACs and DUBTACs difficult, says Shaomeng Wang, a protein degradation expert at the University of Michigan who has been involved with protein-degradation start-ups. Protein-targeting drugs can cause unwanted side effects by degrading or stabilizing proteins in healthy cells as well as unhealthy ones, like cancer cells. And because such bifunctional drugs require two distinct ends, they are relatively large, and it’s difficult to achieve good oral bioavailability and pharmaceutical properties with larger drugs. But Wang notes that orally active PROTACs have made it into the clinic. “It’s challenging,” he says, “but it can be done.”
Sara Buhrlage, a researcher at the Dana Farber Cancer Institute who frequently works with deubiquitinases, agrees drug developers will face challenges in taking DUBTACs into the clinic. But she says the development of PROTACs has laid some groundwork that DUBTACs may be able to take advantage of. “I think it’s going to help that we’re going to be able to take some lessons from that work.”
The news of Vicinitas’ launch comes as Novartis and UC Berkeley announce that they will extend their 5-year-old partnership to drug previously undruggable targets.
“I’m really excited to be working . . . alongside a whole team of drug-hunting Novartis scientists,” Nomura says. “We’re just really, really excited to continue this collaboration and see what it produces.”



Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter