Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Catalysis
Ming Joo Koh
This organic chemist uses common metals to shrink synthetic chemistry’s environmental footprint
by Mark Peplow, special to C&EN
July 15, 2022
| A version of this story appeared in
Volume 100, Issue 25

Credit: Will Ludwig/C&EN/Tim Peacock/Ming Joo Koh
Precious metals like palladium and ruthenium are catalytic mainstays of organic synthesis, but they are also scarce and expensive. Ming Joo Koh—known to everyone as M. J.—is determined to find alternative catalysts that are not only cheaper but also more effective.
“A huge part of my program is to develop nonprecious metal catalysts, mainly from iron or nickel,” says Koh, who started his lab at the National University of Singapore in 2018. “And whatever we do, we have to make sure that it surpasses all previous methods.”
Advertisement
Koh wants to do synthesis in more sustainable ways. “If you’re able to shorten a synthesis by cutting the number of steps, we cut down on the amount of waste that we generate,” he says.
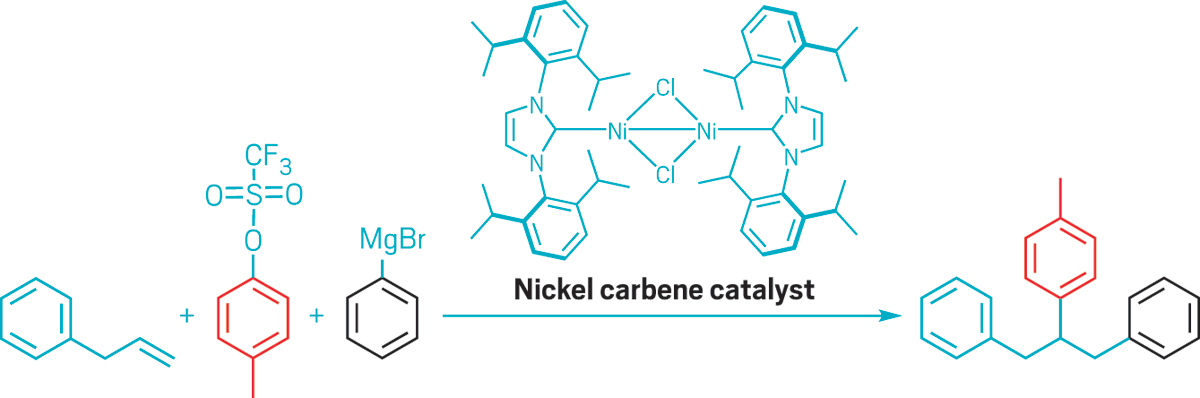
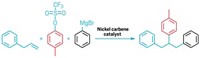
Koh’s team recently showed that a nickel catalyst could provide a useful synthetic shortcut by adding two carbon groups to an unactivated olefin’s C=C bond. Chemists usually need to attach ungainly directing groups to an olefin to guide incoming substituents to the right carbon atoms, but that strategy adds steps to a synthesis and limits the range of products that can be made. In contrast, Koh’s bulky nickel catalyst can do the directing itself and is highly selective, so the incoming groups always end up where he and his chemists want them.
“It’s an enviable piece of work,” says Amir Hoveyda, a chemistry professor at Boston College and Koh’s PhD mentor.
Koh grew up in Singapore, and by the age of 15, he was pretty much hooked on organic chemistry. “It was almost like magic to me when I was young,” he says. Koh’s father and younger sister also work in chemistry, so “perhaps it’s in my genes,” he says.
After undergraduate studies at Nanyang Technological University, Koh joined Hoveyda’s lab, where he worked on olefin metathesis, a ubiquitous reaction that relies on ruthenium or molybdenum catalysts. Metathesis is akin to a molecular square dance that cleaves an olefin’s C=C bond so that the two fragments can form new C=C bonds with different partners.
“His doctoral work took olefin metathesis to a different level,” Hoveyda says. “In my 33 years as a faculty member, out of nearly 200 former group members, no one was as productive.” For example, Koh led the development of a molybdenum metathesis catalyst that produced Z-alkenyl halides, filling a crucial gap in synthetic chemists’ toolbox.
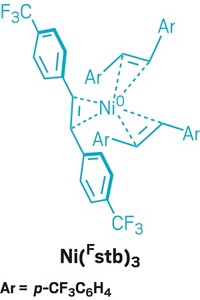
Pivoting from his work with molybdenum to more sustainable metals, Koh is now exploring iron’s ability to catalyze complex organic reactions. “Iron is the most abundant transition metal,” he says. Using iron in place of other transition metals could make catalysis less expensive and more environmentally friendly.
Iron can be fickle, though—it can adopt many oxidation states and spin states, which means chemists may struggle to keep it in its ideal catalytic form. Still, Koh recently made an iron-based catalyst that can form C-glycosides, in which a sugar group is attached to a nonsugar group by a C–C bond. These structures are found in foods and medicines and increasingly attract the pharmaceutical industry’s attention. “Any type of transformation that can modify glycosyl compounds is really, really powerful,” says Michael Schmidt, a scientific director at Bristol Myers Squibb, who was not speaking on behalf of the company.
Koh’s impressive work with nonprecious metal catalysts has caught the eye of Schmidt and other industrial chemists. “These metals are far greener and less expensive for us, and he’s still generating very complex molecules,” Schmidt says. “It’s something we’re very interested in.”
Vitals
Current affiliation: National University of Singapore
Age: 35
PhD alma mater: Boston College
Hometown: Singapore
If I were an element, I'd be: “Iron. It is plentiful and has tremendous potential in sustainable catalysis. The onus is on us to unlock that potential.”
My alternate-universe career: “A horticulturist. I would love to build my own garden of exotic plants.”
See the Talented 12 present their work at a virtual symposium Sept. 19, 20, and 21. Register for free at cenm.ag/t12symposium.

|
|
Precious metals like palladium and ruthenium are catalytic mainstays of organic synthesis, but they are also scarce and expensive. Ming Joo Koh—known to everyone as M. J.—is determined to find alternative catalysts that are not only cheaper but also more effective.
Vitals
▸ Current affiliation: National University of Singapore
▸ Age: 35
▸ PhD alma mater: Boston College
▸ Hometown: Singapore
▸ If I were an element, I’d be: “Iron. It is plentiful and has tremendous potential in sustainable catalysis. The onus is on us to unlock that potential.”
▸ My alternate-universe career: “A horticulturist. I would love to build my own garden of exotic plants.”
“A huge part of my program is to develop nonprecious metal catalysts, mainly from iron or nickel,” says Koh, who started his lab at the National University of Singapore in 2018. “And whatever we do, we have to make sure that it surpasses all previous methods.”
Koh wants to do synthesis in more sustainable ways. “If you’re able to shorten a synthesis by cutting the number of steps, we cut down on the amount of waste that we generate,” he says.
Koh’s team recently showed that a nickel catalyst could provide a useful synthetic shortcut by adding two carbon groups to an unactivated olefin’s C=C bond. Chemists usually need to attach ungainly directing groups to an olefin to guide incoming substituents to the right carbon atoms, but that strategy adds steps to a synthesis and limits the range of products that can be made. In contrast, Koh’s bulky nickel catalyst can do the directing itself and is highly selective, so the incoming groups always end up where he and his chemists want them.
“It’s an enviable piece of work,” says Amir Hoveyda, a chemistry professor at Boston College and Koh’s PhD mentor.
Koh grew up in Singapore, and by the age of 15, he was pretty much hooked on organic chemistry. “It was almost like magic to me when I was young,” he says. Koh’s father and younger sister also work in chemistry, so “perhaps it’s in my genes,” he says.
After undergraduate studies at Nanyang Technological University, Koh joined Hoveyda’s lab, where he worked on olefin metathesis, a ubiquitous reaction that relies on ruthenium or molybdenum catalysts. Metathesis is akin to a molecular square dance that cleaves an olefin’s C=C bond so that the two fragments can form new C=C bonds with different partners.
“His doctoral work took olefin metathesis to a different level,” Hoveyda says. “In my 33 years as a faculty member, out of nearly 200 former group members, no one was as productive.” For example, Koh led the development of a molybdenum metathesis catalyst that produced Z-alkenyl halides, filling a crucial gap in synthetic chemists’ toolbox.
Pivoting from his work with molybdenum to more sustainable metals, Koh is now exploring iron’s ability to catalyze complex organic reactions. “Iron is the most abundant transition metal,” he says. Using iron in place of other transition metals could make catalysis less expensive and more environmentally friendly.
Iron can be fickle, though—it can adopt many oxidation states and spin states, which means chemists may struggle to keep it in its ideal catalytic form. Still, Koh recently made an iron-based catalyst that can form C-glycosides, in which a sugar group is attached to a nonsugar group by a C–C bond. These structures are found in foods and medicines and increasingly attract the pharmaceutical industry’s attention. “Any type of transformation that can modify glycosyl compounds is really, really powerful,” says Michael Schmidt, a scientific director at Bristol Myers Squibb, who was not speaking on behalf of the company.
Koh’s impressive work with nonprecious metal catalysts has caught the eye of Schmidt and other industrial chemists. “These metals are far greener and less expensive for us, and he’s still generating very complex molecules,” Schmidt says. “It’s something we’re very interested in.”



















Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter