Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Synthesis
Companies Tout New Chemo- And Biocatalyst Developments
by Ann M. Thayer
March 13, 2006
| A version of this story appeared in
Volume 84, Issue 11
CATALYSIS
Custom chemical manufacturers continually try to innovate and develop chemistries that will be attractive to customers. New catalysts based on organometallic compounds and biological enzymes stood out among technology developments at the recent Informex exhibition in Orlando, Fla.
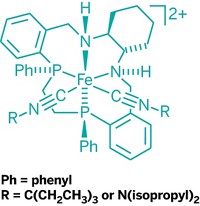
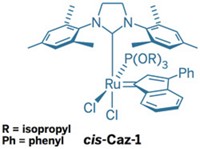
Degussa's homogeneous catalyst business launched catMETium IMesPCy, a new metathesis catalyst. The firm is offering it only for pharmaceutical applications, having taken technology licenses under patents generated at the Technical University in Munich, Germany, by chemistry professor, university president, and Degussa supervisory board member Wolfgang A. Hermann and coworkers, and field-of-use licenses under patents by chemistry professor Steven P. Nolan and others at the University of New Orleans.
The catalyst is an alkylidene ruthenium complex with an N-heterocyclic carbene ligand, similar in structure to ones created by Nobel Laureate Robert H. Grubbs at California Institute of Technology (C&EN, Oct. 10, 2005, page 8). CatMETium performs well in cross-metathesis and in ring-closing, ring-opening, and ene-yne metathesis reactions, all of which are important in pharmaceutical syntheses, says Thomas Reimeier, senior R&D manager for Degussa Homogeneous Catalysts. Catalyst samples are available from Strem Chemicals, while Degussa can supply commercial quantities.
Meanwhile, Solvias has acquired an exclusive license from Yale University for technology to couple aromatic and heteroaromatic chlorides, bromides, and sulfonates with amines and imines. Carbon-nitrogen couplings, such as Buchwald-Hartwig amination reactions, are widely used in fine chemicals production. Yale chemistry professor John F. Hartwig has shown that Solvias' chiral Josiphos ligands, typically used for hydrogenations and a range of other reactions, can be combined with Pd(OAc)2 for C-N coupling.
The new process is economical and industrially viable, Solvias claims, due to high catalyst performance and low catalyst loadings, generally much less than 0.1 mol%. The reaction also has a broad scope, tolerating many functional groups, and offers high yields under mild conditions. Josiphos ligands are already used industrially and thus are available. Solvias and Umicore have just begun offering a sample kit of C-N, as well as C-C, coupling catalysts for screening.
In the biocatalysis area, Kaneka has developed a one-pot enzymatic deracemization process to produce nonnatural l-amino acids. Such amino acids are important building blocks in drug manufacture, says Masahiko Yamada, manager for new product development in Kaneka's fine chemicals division, and have garnered the attention of major pharmaceutical companies.
Kaneka's new process uses four enzymes-a d-amino acid oxidase and l-amino acid dehydrogenase for the actual reaction steps and two others for coenzyme recycling-and gives greater than 90% yield and 99% enantiomeric excess, Yamada says. Because the dehydrogenase is very substrate specific, the company has developed a library of enzymes to handle different amino acids. Yamada says a commercial process is being implemented as the basis for several amino acid projects at the company's Takasago, Japan, site.
Similarly, BASF has developed dehydrogenase biocatalysts to produce optically active styrene oxides and aliphatic alcohols, says John Banger, the firm's manager for new business development in chemicals. The company also has expanded its product portfolio with two new chiral amines, (R,R)- and (S,S)-bis(1-phenylethyl)amine, that can be used in the asymmetric synthesis of nonnatural amino acids or as precursors for chiral bases.
Back to Business
Custom chemical manufacturers showed renewed faith in their industry at this year's Informex
CATALYSIS
Companies Tout New Chemo- And Biocatalyst Developments
Last but not least, Biocatalytics, a Pasadena, Calif.-based enzyme provider, now has the first commercial enzymes for the catalytic reduction of carbon double bonds, says David Rozzell, president and chief executive officer. He calls the ene reductases, or EREDs, remarkably chemoselective, since they reduce only the C=C bond conjugated to an electron-withdrawing group but not other reducible functional groups in the molecule. And with tri- or tetrasubstituted substrates, the enzymes are also highly stereoselective. The company has a 10-enzyme set available for screening.




Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter