Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Materials
Macromolecules Self-Destruct
Adsorption induces carbon-carbon bonds in brushlike macromolecules to break
by Michael Freemantle
March 13, 2006
| A version of this story appeared in
Volume 84, Issue 11

Covalent carbon-carbon bonds in organic molecules are considered tough and difficult to break. It's therefore counterintuitive that the relatively weak attractive forces at play when molecules adsorb to a surface would be strong enough to break these bonds.

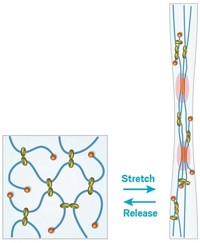
But break they do, according to new research-at least in macromolecules with highly branched architectures. Associate professor of chemistry Sergei S. Sheiko at the University of North Carolina, Chapel Hill; Krzysztof Matyjaszewski, professor of natural sciences at Carnegie Mellon University, Pittsburgh; and their coworkers used atomic force microscopy (AFM) to show the covalent bonds that make up the backbones of brush- like macromolecules spontaneously rupture after adsorption on a substrate (Nature 2006, 440, 191).
The researchers used atom- transfer radical polymerization to prepare polymer backbones with more than 2,000 2-hydroxyethyl methacrylate units and polymeric "bristles" containing as many as 140 or so n-butyl acrylate units. They then deposited the brushlike molecules on the surfaces of various liquid and solid substrates. AFM revealed that the molecules break apart and that the fragmentation rate increases with increasing length of the bristles and the ability of the substrate to attract the side chains.
"Our findings call into question the generally accepted belief that the chemical structures of macromolecules synthesized in solution remain intact after deposition onto a substrate," Sheiko tells C&EN.
He explains that when the molecules adsorb, the side chains spread unevenly over the substrate, predominantly along the polymer backbone. The physical interactions of the side chains with the substrate consequently induce tension that concentrates along the backbone, stretching and eventually snapping it.
"The macromolecule struggles to reconfigure and maximize the number of contacts with the substrate," Sheiko says. "By engaging in this intramolecular tug-of-war, the macromolecule opts to incur the large cost of breaking covalent bonds to reduce the overall free energy of the system."
The rupture of strong, covalent carbon-carbon bonds by the commonplace process of adsorption is a seemingly heretical notion, according to professor of materials science and engineering Steve Granick and research scientist Sung Chul Bae at the University of Illinois, Urbana-Champaign, writing in the same issue of Nature.
They suggest the work could provide a general model for designing materials that have an architecture better able to cope with mechanical stress. The general proof of concept that slow or even forbidden chemical reactions can be activated by mechanical stress is "even more exciting," they add.
In light of the discovery, the surface-induced scission of covalent bonds will need to be carefully considered when complex molecular architectures for surface applications are designed, Sheiko and coauthors note. The phenomenon also opens up "intriguing opportunities" for designing architectures that break at predefined sites, they add.


Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter