Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Policy
Chemical methodology & Library development
October 23, 2006
| A version of this story appeared in
Volume 84, Issue 43
Sept. 18, page 16. Vitamin C was incorrectly identified as citric acid. It is ascorbic acid.
Oct. 9, page 12. The article on brain chemistry should have included the following reference information for the work cited: Science 2006, 314, 130.
I 'd like to offer a few small but significant corrections to Stu Borman's excellent article, "Improving Efficiency" (C&EN, June 19, page 56). On page 69, it says the Chemical Methodology & Library Development (CMLD) centers "are key components of the NIH Roadmap for Medical Research." It's not unreasonable to assume that the CMLD program must be part of the Molecular Libraries Roadmap, since both of these NIH programs deal with chemical libraries. As it happens, the CMLD centers were conceived by the National Institute of General Medical Sciences and are administered and funded solely by NIGMS. In fact, the first CMLD grants were made in 2002, while the road map didn't get off the ground until 2004. NIGMS staff takes pride in having anticipated the thrust of the Molecular Libraries Roadmap by several years.
The same paragraph says that the CMLD centers "are dedicated to the design, synthesis, analysis, and management of such chemical-diversity libraries." In truth, their purpose is to develop new, enabling chemical methodologies having to do with small-molecule libraries. While it is not their explicit mission, the centers do design, synthesize, and manage libraries in the course of validating new methodology.
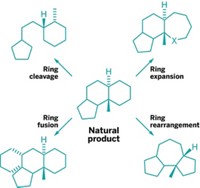
It's also worth noting that diversity-oriented synthesis (DOS) is a term that was coined by Harvard University's Stuart L. Schreiber, and while there are plenty of research groups that target "complex compounds that closely resemble natural products," they don't necessarily categorize their strategies as DOS. To my thinking, DOS has less to do with natural products than with the rapid generation of multiple, highly complex, stereochemically rich molecular frameworks in a single, branched reaction network. The term "natural product-like" has always puzzled me. A compound is either a natural product-it has evolved and persists due to the properties that it confers upon the organism that makes it-or it is not. "Natural product-like" brings to mind another elusive concept: "pregnancy-like."
Overall, I really appreciated this article and its timeliness. The entire industry is in quite a state of flux, and it'll be mighty interesting to see how it all shakes out.
John M. Schwab
Bethesda, Md.


Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter