Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Synthesis
Arenes Hook Up
Catalytic reaction selectively couples benzene and an indole
by Bethany Halford
May 28, 2007
| A version of this story appeared in
Volume 85, Issue 22

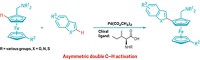
MAKING CERTAIN pharmaceuticals and electronic chemicals could get much simpler, thanks to a new catalytic reaction for coupling two different arenes together.
Coupling one aromatic molecule to another usually requires elaborate functionalization of one or both aromatics beforehand to ensure the compounds react efficiently and selectively with one another. Now, the University of Ottawa's Keith Fagnou and David R. Stuart have devised a way to stick benzene and an N-acetylindole together without functionalizing them first. The reaction proceeds in high yields with high regioselectivity and, most important, produces no self-coupled by-products (Science 2007, 316, 1131).
"The way biaryls are made commercially today, you have to preactivate everything. You can't just couple two carbon atoms together at their C-H bonds," Fagnou explains. The problem is that until now, coupling reactions haven't been selective enough to avoid the stew of homocoupled biaryls that are produced along with the desired cross-coupled biaryl. "How do you get two things to react only with the other coupling partner and not with itself?" Fagnou says. "That was the trick that needed to be solved."
Fagnou and Stuart realized that by selecting aromatic compounds based on nucleophilicity and C-H acidity, they could get one arene to react selectively with the catalyst. The catalyst/arene complex then reacts exclusively with the other arene.
It took a lot of optimization to get the cross-coupling to work, Fagnou and Stuart acknowledge. They had to employ a palladium trifluoroacetate catalyst, along with copper(II) acetate for catalyst regeneration. Critical to the reaction's success were two additives, 3-nitropyridine and cesium pivalate, and the pivalic acid solvent.
The new process "could have immense practical importance for the synthesis of materials, electronic devices, and drugs," writes Jonathan A. Ellman of the University of California, Berkeley, in a Science commentary. "However, further advances will be required to enhance reaction efficiency, for example, by reducing catalyst and terminal oxidant loading levels."


Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter