Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Environment
Mineral Structure Solved
May 28, 2007
| A version of this story appeared in
Volume 85, Issue 22
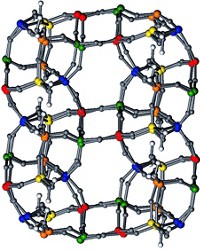
Ferrihydrite is a naturally occurring iron oxide and is produced commercially to scavenge heavy metals from wastewater. But there has been no consensus on ferrihydrite's formula or structure because its nanosized crystallites have foiled traditional structure determination methods. Now, geochemists F. Marc Michel, Martin A. A. Schoonen, and John B. Parise at the State University of New York, Stony Brook, and colleagues propose a new atomic model for ferrihydrite (Science, DOI: 10.1126/science.1142525.) They collected X-ray scattering data at a synchrotron source, then analyzed the data with an atomic pair distribution function method. The researchers determined that in its ideal form, ferrihydrite has the formula Fe10O14(OH)2 and consists of 20% FeO4 tetrahedra (shown in red, with oxygen in blue) and 80% FeO6 octahedra. "The work represents a major leap forward in understanding this important mineral," says chemist R. Lee Penn of the University of Minnesota in an accompanying Science commentary.


Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter