Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Materials
Chaining Up Fullerenes
December 10, 2007
| A version of this story appeared in
Volume 85, Issue 50
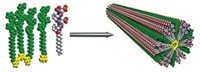
Supramolecular polymers are chains of monomeric compounds that self-assemble through reversible interactions, such as hydrogen bonding or metal coordination. Now, a group led by Nazario Martín at Madrid's Complutense University has exploited π-π electronic interactions to assemble a supramolecular chain of C60 molecules tethered together via a tetrathiafulvalene analog called exTTF (Angew. Chem. Int. Ed., DOI: 10.1002/anie.200703049). Attached to one C60, two exTTF molecules linked by a spacer group form a concave aromatic surface that recognizes the convex surface of a second C60 with an attached exTTF system. The researchers observed chains (shown) of up to five repeat units by MALDI-TOF mass spectrometry. Molecular weight estimates from dynamic light-scattering experiments indicate that chains with up to 400 repeat units could be forming, although the largest structures might be due to oligomer aggregation, the researchers note. Electronically, exTTF is an electron donor, while C60 is an electron acceptor. Thus, the polymers may be useful in optoelectronic devices, particularly in organic photovoltaic solar cells.



Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter