Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
People
ACS Award In Industrial Chemistry
Recipients are honored for contributions of major significance to chemistry
by Michael McCoy
January 7, 2008
| A version of this story appeared in
Volume 86, Issue 1
Sponsored by the ACS Division of Business Development & Management
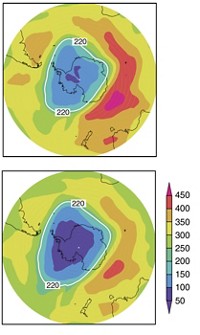
A car company might seem an unlikely source of world-changing chemistry, yet it was out of the Ford Motor Co. laboratories of atmospheric chemist Timothy J. Wallington that proof came some 15 years ago that a key chlorofluorocarbon (CFC) replacement did not threaten the ozone layer.
Wallington, 49, is a technical leader at Ford's Dearborn, Mich., research center. An Englishman who lives near Detroit, he earned a bachelor's degree and Ph.D. in physical chemistry at Oxford University's Corpus Christi College.
Growing up, Wallington says, he thought of the U.S. as "a place to do really great science." He got the chance to become a part of that community in 1984 when he landed a postdoc position at the University of California, Riverside.
He joined Ford three years later and has worked there ever since, studying the atmospheric chemistry of industrially important organic compounds. "The environment and the people here are excellent," Wallington says. "It's a bit of a well-kept secret, but Ford has a strong environmental chemistry effort."
Scientists first recognized in the 1970s that CFCs and their degradation products were accumulating in the atmosphere to the possible detriment of the ozone layer. Although the connection was unproven at time, Ford was intensely interested because the company used large amounts of CFC-12 to air condition its cars and trucks.
Chemical companies such as DuPont began proposing numerous potential CFC replacements, and Wallington was able to show that one of them, hydrofluorocarbon (HFC)-134a, did not create toxic degradation products and did not pose any risk to stratospheric ozone.
HFC-134a went on to become the standard refrigerant used in auto air conditioners. Today, ironically, it is under fire in Europe as a contributor to global warming. "In Europe we find ourselves in the position of replacing the replacements," Wallington observes.
Wallington's decision to work in industry doesn't mean he has forgotten his academic roots. Soon after he joined the company, he began hosting environmental chemistry graduate students-notably ones from the University of Copenhagen, University of Toronto, and Kyoto University-in Ford's Dearborn labs. He says he finds the interaction with students satisfying, as they often bring new and unique perspectives to the science.
And although Wallington's research is conducted with Ford's goals in mind, he doesn't see himself as scientifically constrained. "We have the freedom and encouragement to study things from a broader perspective than one might expect," he says. "The beautiful thing about chemistry is that everything is related to everything else." For example, he is bringing a longtime interest in oxygenated compounds to bear on the study of ethanol and other recently developed biofuels.
Indeed, according to James A. Franklin, a former researcher with the fluorocarbon producer Solvay and now managing director of CLF-Chem Consulting in Brussels, a wide-ranging approach to environmental chemistry is one of Wallington's hallmarks.
Franklin worked during the 1990s on a joint research program sponsored by fluorocarbon companies worldwide. What struck Franklin at the time was the breadth of Wallington's work.
At first glance, he says, it might have appeared that many of the compounds Wallington studied were of no interest to Ford. But Franklin realized that investigating whole compound families allowed Wallington and his coworkers to make predictions by probing how rate constants and mechanisms change with variations in structure. "Both Wallington and Ford management must be praised for this fundamental, unifying approach-the hallmark of good science," he says.
Wallington will present the award address before the Division of Business Development & Management.




Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter