Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Environment
Fast Photochromism
July 7, 2008
| A version of this story appeared in
Volume 86, Issue 27
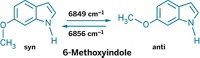
Bespectacled folks once marveled at lenses that darken in sunlight and return to their untinted state indoors, but the sluggish transition from tinted to transparent left some feeling less than enlightened. Scientists are now getting closer to lenses that shift in an instant, thanks to a new photochromic compound. Jiro Abe and coworkers at Japan's Aoyama Gakuin University have created a hexaarylbiimidazole derivative that changes from colorless to moss green when irradiated with UV light (Org. Lett., DOI: 10.1021/ol801135g). The light homolytically cleaves the C–N bond that links the molecule's imidazole rings, generating a pair of 2,4,5-triphenylimidazolyl radicals (shown). A naphthalene moiety tethers these two radicals together, thereby preventing them from diffusing away from one another. In the absence of UV light, the radicals quickly recombine. At room temperature, this rapid thermal bleaching returns the molecule to its colorless form in a matter of milliseconds. The coloring and decoloring process is so fast that Abe's team can use a UV-light-emitting diode to make a green-colored cloud zip around a solution of the compound.



Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter