Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Synthesis
A New Take On Vibrational Excitation
July 7, 2008
| A version of this story appeared in
Volume 86, Issue 27
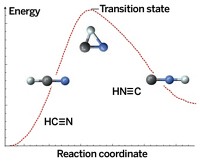
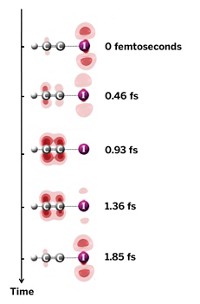
A previously unknown mechanism describing vibrational excitation of molecules undergoing collisions may play a role in all collisions between pairs of neutral species that have the potential to form chemical bonds with one another, according to a new study (Nature 2008, 454, 88). In collisions between a hydrogen atom and a hydrogen molecule, for example, some of the kinetic energy associated with the atom's and the molecule's translational energy can be converted to vibrational and rotational energy in the molecule. Conventional wisdom holds that the energy-transfer process in this classic textbook reaction proceeds by way of a head-on collision between the atom and molecule (shown at top). The event sends the colliding partners rebounding in opposite directions, with the atom being backscattered along the path of its initial approach and away from the now-excited molecule. According to the new mechanism (bottom), which was proposed by Stuart J. Greaves of the University of Bristol, in England; Richard N. Zare of Stanford University; and coworkers, glancing collisions can lead to formation of a transitory bond between the atom and molecule. That process stretches the molecule and renders it vibrationally excited. When the momentary bond breaks, the atom continues along its initial path.



Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter