Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Business
U.S. Probe Hurts Ranbaxy
Questions about quality spark concerns over buyout
by Melody Voith
July 21, 2008
| A version of this story appeared in
Volume 86, Issue 29

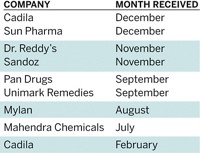
INDIA'S RANBAXY Laboratories, one of the world's largest makers of generic drugs, faces investigation by the U.S. Justice Department into whether it violated FDA regulations by manufacturing adulterated and misbranded drugs.
The allegations may disrupt Japanese drug firm Daiichi Sankyo's plans, announced on June 11, to buy a majority stake in the company for $4.6 billion (C&EN, June 16, page 14). News of the investigation prompted a 12% drop in Ranbaxy's stock price.
DOJ is investigating because more than 25% of Ranbaxy's generics are sold in the U.S. The firm also supplies generic versions of antiretroviral medicines for President George W. Bush's emergency program for AIDS relief, which provides low-cost HIV drugs to patients in developing countries.
In its July 3 filing at the U.S. District Court for the District of Maryland, DOJ alleges that Ranbaxy submitted "false and fabricated" information to FDA about stability and bioequivalence and attempted to conceal violations of current Good Manufacturing Practices.
According to the filing, "evidence suggests that Ranbaxy uses [active pharmaceutical ingredients] from unapproved sources, blends unapproved API with approved API, and uses less API in its drug than had been approved by the FDA" and that these conditions may lead to a drug that is "subpotent, superpotent, or adulterated."
In a public statement, Ranbaxy "strongly denies the allegations contained in the motion" and points out that no legal charges have been filed against the company. Ranbaxy says that it "has agreed to produce the specific documents sought by the motion."
Rich Salem, head of public affairs for Daiichi, tells C&EN that "we will follow this matter very closely as additional facts become public. Our acquisition plans are unchanged at this time."
The government allegations against Ranbaxy stem from a 2006 FDA investigation of its plant in Paonta Sahib, India, that resulted in a warning letter outlining significant violations of FDA regulations and specifying a hold on drugs originating from that facility.


Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter