Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Synthesis
Making Borosilicate Nanoparticles Is Now Possible
September 15, 2008
| A version of this story appeared in
Volume 86, Issue 37
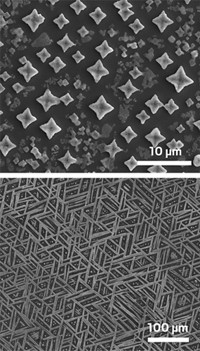
A team of researchers at the Swiss Federal Institute of Technology, Lausanne, reports the first synthesis and characterization of borosilicate nanoparticles (Nat. Nanotechnol., DOI: 10.1038/nnano.2008.262). Borosilicate glass is inert, and as such it can withstand high temperatures and harsh chemical conditions, unlike polymers or silica used to make nanoparticles. Until now, borosilicate microparticles could be formed from a glass melt, but an unstable boron oxide precursor made the fabrication of nanoparticles impossible. Virendra K. Parashar, Martin A. M. Gijs, and colleagues got around this problem by first preparing a borosilicate gel from tetraethylorthosilicate and trimethoxyboroxine, using formic acid as a catalyst and dichloromethane and 2-propanol as solvents. Exposing a droplet of the gel to water without prior contact with air incites a dynamic reaction that immediately forms the solid nanoparticles. The researchers characterized the particles, which range from 100 to 500 nm in size, by microscopy techniques and elucidated the exothermic phase-separation mechanism that forms the nanoparticles via NMR studies. The researchers say the availability of these particles will broaden the potential of nanoparticles for chemical uses, including applications in filtration membranes, optics, and medicine.
Click her to see Injection by a capillary of borosilicate sol in water

Click her to see Injection by a capillary of borosilicate sol in water




Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter