Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Materials
Transpiration Mimic
'Synthetic tree' pumps water with negative pressure
by Celia Henry Arnaud
September 15, 2008
| A version of this story appeared in
Volume 86, Issue 37

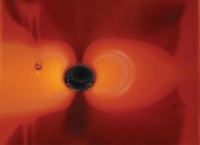
SCIENTISTS AT CORNELL UNIVERSITY have created a "synthetic tree" microfluidic device that mimics transpiration, the process by which a plant pulls water through its roots and up to its leaves.
In transpiration, evaporation of water through the leaves creates a large negative pressure (tension in the liquid) that drives the process. The analogous large negative pressures generated in the microfluidic device suggest that other processes requiring large pressure differences, such as high-performance liquid chromatography, could be performed in microfluidic devices without external pumps.

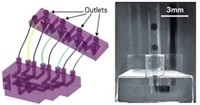
Assistant professor of chemical and biomolecular engineering Abraham D. Stroock and graduate student Tobias D. Wheeler defined the minimum requirements for transpiration as a water-filled conduit coupled to its surroundings by two membranes. The challenge, Stroock says, was finding the right material for the membranes that separate the bulk water inside the plant from the water in the atmosphere or soil.
"The breakthrough was finding the material that could place the liquid in equilibrium with subsaturated phases of water," he says. "That's the step that generates these negative pressures and drives the liquid through the so-called plant."
The breakthrough material turned out to be a poly(hydroxyethyl methacrylate) hydrogel. From this hydrogel the team built synthetic trees consisting of "leaf" and "root" networks, each with 80 microchannels of various lengths arranged in a circle, connected by a single "trunk" microchannel (Nature 2008, 455, 208).
"For simplicity, we built this entire network from the root region through the trunk to the leaf region all in a single material," Stroock says. "The architecture allows us to separate the root from the environment of the leaf."
Because they are separated, the leaf and root networks can experience different water environments. Plant roots are typically in a wetter environment than plant leaves. The water then spontaneously follows the chemical potential gradient from the roots to the leaves. Stroock and Wheeler achieved pressures in the microdevice of about −70 atm.
Under these conditions the water is metastable, meaning that it is prone to vaporization. "Our method opens up a new route to study the metastable state of liquids under tension," Stroock says. "Even for water, the world's most studied substance, the most basic thermodynamic and kinetic properties have never been mapped in this regime."
The work is a "significant development," says Pablo G. Debenedetti, professor of chemical engineering at Princeton University. "It shows how, taking inspiration from nature, it is possible to engineer the reliable, safe, and efficient handling of metastable liquids. The work is remarkable because of the very satisfying way in which it combines a solid grounding in thermodynamics, appreciation for the mechanism of water transport in vascular plants, and clever selection and microfabrication of a synthetic material so as to mimic the action of a tree's root, xylem, and leaves."



Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter