Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Synthesis
Bryostatin, Faster
Highly selective catalysis dramatically reduces steps to multiring structure
by Carmen Drahl
December 1, 2008
| A version of this story appeared in
Volume 86, Issue 48
ALKYNES' UNIQUE EMBRACE of transition-metal catalysts has enabled the shortest route yet to the bryostatins, marine natural products with promising anticancer and memory-enhancing properties. The synthetic advance, reported in Nature (2008, 456, 485), could allow a more thorough evaluation of the compounds' biomedical potential.
Because only small amounts of the bryostatins are available, investigations of their therapeutic activity have been hampered. Three previous total syntheses of bryostatin family members have been reported, but they required lengthy sequences of at least 40 consecutive synthetic steps. Now, chemists Guangbin Dong and Barry M. Trost of Stanford University have developed a route to a bryostatin that clocks in at 26 steps.
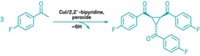
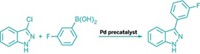
To make bryostatin 16, Dong and Trost implemented a series of transition-metal-catalyzed reactions, each of which relies on the metal's selective interaction with an alkyne over other functional groups. "The alkynes play a critical role in allowing us to reduce the number of steps" by, for instance, reducing the need for protecting groups, Trost explains. Furthermore, the key reactions adhere to Trost's philosophy of atom economy, in which all the atoms in the reactants wind up in the products.
In a crucial step, the Stanford team used a palladium-catalyzed coupling of two alkynes to form a 22-membered ring intermediate. This marks the first time that such a large ring structure has been made by this type of carbon-carbon bond-forming reaction, writes University of Montreal chemist André B. Charette in a commentary accompanying the report.
The Pd-catalyzed reaction allowed the team to avoid using alkene metathesis to make the large ring, a strategy that had failed them in the past. It also set the stage for a gold-catalyzed reaction that harnessed alkyne reactivity to selectively form a six-membered dihydropyran ring instead of a typically easier to form five-membered ring. The team now plans to use the route to make additional bryostatins, as well as analogs.
"This is a monumental piece of work," says organometallic chemist Alois Fürstner, a director at the Max Planck Institute for Coal Research, in Mülheim an der Ruhr, Germany. The team's creative handling of delicate selectivity issues makes the synthesis highly impressive, he adds.



Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter