Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Synthesis
Acetaldehyde In Action
Troublesome reagent coaxed into organocatalyzed, enantioselective reactions
by Bethany Halford
February 25, 2008
| A version of this story appeared in
Volume 86, Issue 8
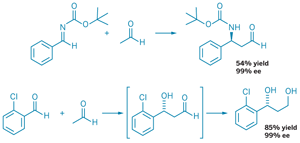
Acetaldehyde can act as a nucleophile in organocatalyzed, enantioselective Mannich (top) and crossed-aldol (bottom) reactions with good yields and high enantiomeric excess (ee).
DESPITE ITS VALUE as a nucleophile, the simple molecule acetaldehyde has long been considered too problematic to use in enantioselective reactions catalyzed by small molecules. Now, thanks to tweaking of reaction conditions, two groups have managed to get this finicky aldehyde to work well in organocatalyzed carbon-carbon bond-forming reactions. The finding offers synthetic chemists a new tool for constructing all manner of molecules.
"These two reports are the first instances where acetaldehyde has been successfully engaged" in organocatalyzed, enantioselective Mannich and aldol reactions, comments David A. Evans, a chemistry professor at Harvard University. The two-carbon aldehyde tends to be troublesome because it reacts with itself to form oligomers and polymers, and its reaction products, which are also unbranched aldehydes, will compete with acetaldehyde to give unwanted by-products.
Benjamin List and colleagues at the Max Planck Institute in Mülheim, Germany, found they could get acetaldehyde to work as a nucleophile in a proline-catalyzed Mannich reaction-wherein an aldehyde is coupled with an imine-by adding a five- to 10-fold excess of the reagent (Nature, DOI: 10.1038/nature06740). "With a higher acetaldehyde concentration, the competition between the initial reaction product and acetaldehyde for the imine electrophile should be in favor of acetaldehyde, simply because there is more of it," List explains.
The reaction works with both aromatic and aliphatic imines to produce β-aminoaldehydes in reasonable yields and with high enantioselectivities. "We have shown these compounds to be extremely valuable in the synthesis of biologically active molecules and also new drug entities," List notes.
Similarly, by using a fivefold excess of acetaldehyde and a bulky trifluoromethyl-substituted diarylprolinol catalyst, Yujiro Hayashi and coworkers at Japan's Tokyo University of Science report the first direct, enantioselective crossed-aldol reaction of acetaldehyde (Angew. Chem. Int. Ed., DOI: 10.1002/anie.200704870). The crossed-aldol reaction works with both aromatic and olefinic aldehydes, Hayashi's group reports.
Because the resulting aldol products are unstable, Hayashi's group reduced the compounds to the corresponding diols before calculating yields and enantioselectivities, which were, respectively, good and excellent. The researchers believe the stereoselectivity is dictated by hydrogen bonding between the electrophilic aldehyde and the enamine nucleophile formed by the acetaldehyde and the diarylprolinol catalyst.
Both List and Hayashi believe the newfound usefulness of acetaldehyde in organocatalyzed, enantioselective reactions will have numerous applications in organic synthesis.


Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter