Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Physical Chemistry
Mysterious Attraction
New explanation for hydrocarbon stability invokes 'protobranching'
by Elizabeth K. Wilson
February 25, 2008
| A version of this story appeared in
Volume 86, Issue 8
PAUL VON RAGUÉ SCHLEYER says the idea hit him while teaching a graduate class on the structure and energy of organic molecules: The mechanism that irrefutably stabilizes branched alkanes should stabilize linear alkanes as well.
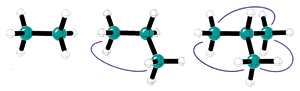
Alkyl-alkyl interactions (blue lines) stabilize alkanes, according to the protobranching concept.
The known stabilizing effect in branched alkanes, which Schleyer attributes to attractive interactions between alternating carbons and their attached hydrogens (1,3 interactions), would certainly be weaker in unbranched alkanes, but Schleyer speculated it would be present in the unbranched compounds just the same.
This new model of unbranched alkane interactions is controversial because it requires changing energetics calculations for almost every hydrocarbon and rethinking what stabilizes them. The idea is about as radical as when Nobel Laureate Robert S. Mulliken proposed hyperconjugation (orbital delocalization) as an explanation for trends in C–H bond strengths in the 1930s.
The new concept, dubbed "protobranching," was developed by Schleyer, a chemistry professor at the University of Georgia, Athens; University of California, Los Angeles, chemistry professor Kendall N. Houk; and their colleagues (Chem. Eur. J. 2007, 13, 7731).
The name stems from the idea that each 1,3 interaction is a "prototypical branch." Thus, isobutane has three protobranches, while butane has two, and neopentane has six, while methane and ethane have none.
Protobranching effects, when included in hydrocarbon energy calculations, resolve a host of inconsistencies in energies that have plagued hydrocarbon thermodynamics for decades. For example, wildly differing experimental values that are often obtained for properties of benzene, such as resonance energy and aromatic stabilization, suddenly come into alignment when protobranching is taken into account.
The concept joins other energetic perturbations that affect bond stability, such as conjugation, ring strain, and aromaticity. The mechanism for protobranching's 1,3 attractions remains unknown, but Schleyer, Houk, and coworkers believe it's related to the physical property of electron correlation-the complicated motions of electrons as they try to stay out of each other's way.
"I think it's wonderfully provocative," Yale University organic chemistry professor William L. Jorgensen says of the new concept. "I'm glad to see that people are still worrying about these things."
However, the protobranching concept squares off against another recent theory that suggests the exact opposite: that 1,3 interactions are repulsive. Virginia Commonwealth University chemistry professor Scott Gronert has proposed that alkane and alkyl radical energetics can be accurately reproduced by considering 1,3 repulsions caused by steric strain, a phenomenon he calls geminal repulsion (C&EN, March 6, 2006, page 65).
THE ARGUMENT "is getting at the bedrock of what organic chemists think is truth," says Gronert, who has submitted a protobranching rebuttal to Chemistry: A European Journal.
"The problem is a matter of which data feel more important," notes Steven M. Bachrach, chair of the chemistry department at Trinity University in San Antonio, Texas, and author of the textbook "Computational Organic Chemistry" and a blog of the same name (hackberry.chem.trinity.edu/blog/). "How do you weigh all different competing data and interpretations?"
The debate is likely to continue. In fact, Andreas Zavitsas, chemistry professor at Long Island University, and colleagues have now submitted a paper to the Journal of Physical Chemistry A with still another method for calculating hydrocarbon energies, one that does not invoke either protobranching or geminal repulsion.
Instead, they multiply the numbers of primary, secondary, and tertiary hydrogens by constants to get heats of formation that match experimental values. "Schleyer says 1,3 interactions are attractive, Gronert says 1,3 interactions are repulsive," Zavitsas tells C&EN. "We say we don't need either."
The debate attests to an ongoing renaissance in hydrocarbon thermodynamics studies. With new capabilities of theory and experiment—in particular the recent discovery that density functional theory, a ubiquitous computational tool, performs poorly for many hydrocarbons—chemists have recently been reexamining old ideas.
The tried-and-true method for assessing a molecule's stability has been to compare the heat of formation of a molecule affected by conjugation or ring strain with that of a reference compound that's not. But Schleyer, Houk, and coworkers say it's unfair to compare the energies of molecules that "benefit" from protobranching to different extents. For example, strain-free cyclohexane, commonly used as a reference molecule, is stabilized by six protobranches, which reduce its energy and throw off comparisons with compounds that have fewer protobranches.
All these models fit experimental data well. So which is right? That may be a difficult question to answer, say those involved. "Some of these things cannot be proven or disproved," Houk says. "It's a matter of what model is more generally useful in understanding hydrocarbon stabilities."


Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter