Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Synthesis
New Way To Protect Unstable Boron Reagents
Masked boronates make 2-pyridyl coupling possible
by Stu Borman
May 11, 2009
| A version of this story appeared in
Volume 87, Issue 19

A NEW CARBON-CARBON coupling technique provides a route to compounds that are currently difficult to synthesize. The approach eases access to 2-pyridyl and other heterocyclic derivatives that are of key importance, especially in drug discovery, but have been particularly challenging synthetically.
Chemists often form C–C links by using Stille or Suzuki coupling reactions. But Stille reactions use toxic tin reagents, and Suzuki reactions use boronic acid intermediates that tend to be unstable. Extensive efforts have been made to reduce the instability of Suzuki boronates, but most approaches have major limitations.
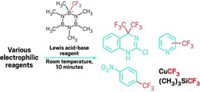
One of the most promising tactics has been the use of stable organotrifluoroborates in place of unstable boronates, an approach developed in the past few years by organic chemistry professor Gary A. Molander and coworkers at the University of Pennsylvania and elsewhere. But the trifluoroborate technique still doesn't work well in some cases, such as with 2-pyridyl couplings.
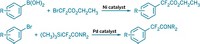
Now, grad students David M. Knapp and Eric P. Gillis and assistant professor of chemistry Martin D. Burke of the University of Illinois, Urbana-Champaign, have further expanded Suzuki coupling by developing a method that uses N-methyliminodiacetic acid (MIDA) to protect unstable boronates (J. Am. Chem. Soc., DOI: 10.1021/ja901416p). MIDA boronates are easy to synthesize and stable, and they release unstable boronic acid intermediates slowly, enabling Suzuki coupling to occur before the intermediates decompose. The technique makes it feasible to carry out 2-pyridyl and other heterocyclic couplings.
Lawrence G. Hamann, executive director for Global Discovery Chemistry at Novartis, in Cambridge, Mass., comments that MIDA boronates tolerate a substantial range of reaction types and that 2-pyridyl MIDA boronates in particular "are a major advance, as the trifluoroborate methodology does not work here." He notes that 2-pyridyl moieties are "ubiquitous in many druglike molecules."
"MIDA boronate stability, reliability, and ease of use, in conjunction with the ability to mitigate the use of toxic tin-derived reagents, will accelerate the broad incorporation of this tool into synthetic strategies," adds Peter T. Meinke, senior director of medicinal chemistry at Merck & Co., in Rahway, N.J.



Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter