Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Policy
Nanotechnology Investment
U.S. Focuses on commercialization and strengthening environmental, health, and safety research
by Britt E. Erickson
April 12, 2010
| A version of this story appeared in
Volume 88, Issue 15

The U.S. remains the world’s leader in nanotechnology, but that position is being threatened by China, South Korea, and the European Union, according to a report released last month by the President’s Council of Advisors on Science & Technology (PCAST).
The report, which was prepared for the White House and Congress, recommends several changes to the government’s interagency program that coordinates federally funded nanotechnology R&D, the National Nanotechnology Initiative (NNI). The changes aim to ensure that the U.S. continues to dominate the field over the next decade.
Top recommendations of the report include increasing investments in nanomanufacturing and product commercialization so that novel nanotech products enter the marketplace, increasing the number of workers with expertise in nanofabrication, and strengthening commitments to environmental, health, and safety (EHS) research.
The priorities outlined in the PCAST report are the same areas that many different stakeholders—including the government itself—are already actively discussing. They are the focus of legislation working its way through Congress, and they are of great concern to the nanotech industry, which held a two-day gathering in Washington, D.C., last month to meet with congressmen and federal regulators. Also in March, NNI wrapped up a series of workshops to address gaps in EHS research.
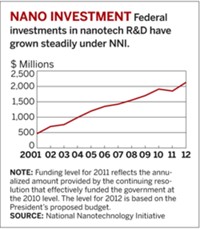
NNI was created in 2001, with eight participating agencies and an annual budget of $464 million. Today, 25 agencies participate in the initiative. Of those agencies, 14 contribute to its funding, which for fiscal 2011 is proposed to total $1.8 billion.
Over the past decade, NNI has provided about $12 billion for nanotech R&D, which has had a “catalytic and substantial impact” on U.S. nanotechnology growth, the PCAST report concludes. However, the report recommends that NNI increase its investment, particularly in the area of nanomanufacturing, to ensure that the U.S. keeps its leadership position. The U.S. has lagged behind Asia and the EU in terms of government funding for nanotech R&D since 2006, according to data from Lux Research, a research and advisory firm specializing in emerging technologies.
“Our early investments in nanotechnology have brought us to the point where the science is being translated into important new products in health, electronics, energy, defense, and other fields,” Maxine L. Savitz, cochair of PCAST’s NNI Working Group and vice president of the National Academy of Engineering, noted in a statement. “Going forward we need to place even more emphasis on the commercialization of the technology—through, for example, strategic funding of nanomanufacturing,” she says.
The report recommends that NNI increase its investment in nanomanufacturing by 100% over the next five years. In 2011, government funding for nanomanufacturing is proposed to make up only 5.8% of the total NNI budget.
Along with nanomanufacturing comes responsible product development and understanding the risks of a product. The report recommends a more rationalized approach to identifying the risks of nanotechnology and developing a cross-agency strategic research plan to fill EHS knowledge gaps.
“It is important not only to continue increasing the federal investment in environmental, health, and safety research but to do so in a coordinated way so the most important questions are answered first,” said Ed Penhoet, cochair of PCAST’s NNI Working Group and director of Alta Partners, a venture capital firm. “That approach will ensure safety, bolster public confidence, and provide a clear path to market for new companies and their products.”
Another key recommendation in the report is for Congress and the Obama Administration to take steps to provide permanent resident status for foreign individuals who hold an advanced degree in science and engineering from an accredited institution and are employed within the U.S. in that discipline. The report points to the lack of a skilled American workforce in nanotechnology, adding that one-third of foreign individuals trained in the U.S. return to live in their native countries.
Some of the PCAST recommendations were also brought up by members of the NanoBusiness Alliance, an industry trade association dedicated to commercializing nanotechnology, during a congressional briefing on March 16. The group was in town for its 9th Annual Washington, D.C., Roundtable Event, which included visits with key senators to discuss nanotechnology legislation.
The nanotech industry, along with many other stakeholders, is particularly interested in seeing Congress pass the NNI Amendments Act of 2009. The bill shifts priorities away from basic research toward activities that encourage commercialization of nanotech products and lead to economic benefits. It also requires all federal agencies that participate in NNI to develop a plan for EHS research. The bill passed the House of Representatives in February 2009, but it has yet to move in the Senate.
To help build momentum for the bill in the Senate, representatives from several companies spoke at the March 16 briefing, hosted by Sen. Ronald L. Wyden (D-Ore.) and the Congressional Nanotechnology Caucus. The representatives provided members of Congress and staffers with information about nanotech products under development and how nanotechnology helps create jobs. They also took the opportunity to let Congress know what keeps them up at night.
High on the nanotech industry’s list of concerns are the lack of Americans with training in nanotechnology, the lack of H-1B visas that allow foreigners to work in the U.S., and the lack of access to capital in the $5 million to $100 million range. Such capital is needed for nanotech R&D and product development, but has dried up because of uncertainty about the risks of nanotechnology.
Several industry representatives at the briefing emphasized the need for training people at the technician level in nanomanufacturing. There are plenty of people with Ph.D.s that have expertise in nanotechnology, but they don’t run the machines, said James M. Hussey, chief executive officer of NanoInk, an Illinois-based company that specializes in nanotechnology manufacturing and applications for the life sciences and semiconductor industries. What’s really needed, he added, are lower level technicians who know how to use the necessary nanomanufacturing equipment.
Because of the lack of U.S. workers with nanomanufacturing expertise, and the difficulty in obtaining H-1B visas for foreign workers with such expertise, NanoInk had to open a small lab in Cambridge, England, to get the skilled workforce it needs, Hussey stressed.
While in Washington, members of the NanoBusiness Alliance also met with federal regulators to stimulate dialogue and find out the latest on regulatory actions regarding nanotechnology.
At that meeting, industry representatives expressed frustration with the current regulatory system because various offices within a single agency regulate nanotechnology differently and one office doesn’t seem to know what another is doing.
For example, the Environmental Protection Agency regulates nanotechnology by two different laws—the Federal Fungicide, Insecticide & Rodenticide Act (FIFRA) and the Toxic Substances Control Act (TSCA)—which are managed under two different offices. So when an antimicrobial claim is made on a nanotech product, such as those containing nanosilver, the product is regulated under FIFRA and handled by EPA’s pesticides office. But for other nanoscale materials, such as carbon nanotubes and graphene, regulation is handled by EPA’s toxics office under TSCA.
A similar situation exists at the Food & Drug Administration, where regulation of nanoscale materials is handled by a variety of offices. For instance, depending on a product’s intended use, it may fall to the agency’s food, cosmetic, or drug center for evaluation.
Industry wants the agencies to come up with a more streamlined approach to regulating nanotechnology because it is difficult to navigate the current system. Some company representatives questioned how regulators will handle multifunctional products, such as those that use nanotechnology for imaging as well as therapeutics. Such products are coming “fast and furious,” one participant noted.
A clear and robust regulatory process is also important, according to industry representatives, to reduce the uncertainty around their products. The concern is that the longer it takes for regulatory agencies to harmonize their processes, the more the industry will move offshore because investors are willing to fund development of products with such high uncertainty.
Frustrations about regulation aside, industry representatives agreed that it is in their best interests to proactively address the risks of nanotechnology. Assessing the risks of nanotechnology is difficult, however, because in most cases toxicity data on nanomaterials are insufficient.
Although government investment in EHS research has grown from $35 million in 2005 to a proposed $117 million in 2011, several knowledge gaps still remain. And, although there are calls like the one in the PCAST report for more investment in this area, funding for EHS research still only makes up a small percentage (6.6% in 2011) of the total NNI budget.
To help identify those knowledge gaps and prioritize nanotech EHS research needs, NNI convened a series of four workshops over the past year. The first workshop focused on exposure assessment, the second on environmental impacts, and the third on human health effects. The second and third workshops also examined the need for new instrumentation, metrology, and analytical methods. The final workshop, held at the end of March, focused on risk management methods and the ethical, social, and legal implications of nanotechnology.
NNI plans to use the information gathered during the workshops as it develops a new strategic plan for EHS research. Its current strategy, released in February 2008, was heavily criticized by stakeholders, including industry trade groups, nanomaterial manufacturers, and environmental organizations.
In late 2008, the National Research Council released a report that blasted NNI’s EHS research strategy, saying it lacks a clear vision, specific goals, and an evaluation of the current state of the science. The report recommended that NNI hold a series of meetings to gather broader stakeholder input about EHS data gaps, and that is just what NNI did.
NNI is now trying to make sense of all the information it gleaned from the four EHS workshops. Some of the biggest challenges pointed out by participants include the lack of characterization of nanomaterials used in toxicology studies and the need for better models to predict the toxicity of nanomaterials.
There was also a lot of talk about designing nanomaterials to be less toxic, but, as some participants noted, that is not always possible. For example, nanoscale titanium dioxide, an ingredient found in many sunscreens, generates reactive oxygen species. “You can’t design away that property,” Gregory V. Lowry, professor of civil and environmental engineering at Carnegie Mellon University, stressed at the March workshop. In such cases, he said, one should eliminate the potential for exposure by coating nanoparticles with aluminum oxide or using protective equipment during manufacturing.
Kristen Kulinowski, director of the International Council on Nanotechnology (ICON) at Rice University, pointed out that in the scientific literature hazard data far outstrip exposure data for nanomaterials. As a result, she said, there has been an imbalance in the discussion, with few people asking, can we be exposed?
Worker safety is another issue that Kulinowski is concerned about. She noted that there is limited work in the literature on nanomaterial toxicity that is relevant to occupational settings. As a result, ICON has teamed up with several other organizations to launch the GoodNanoGuide (goodnanoguide.org), an online interactive forum for exchanging ideas on best practices for handling nanomaterials in the workplace, including university laboratories.
Many other participants at the March workshop agreed that more needs to be done to train chemists and engineers in toxicology so they better understand the EHS risks of the materials they are working with. But incorporating such a change into a chemistry department’s curriculum would have to involve accreditation bodies, such as the American Chemical Society. And those conversations have yet to begin.
In the meantime, NNI appears to have gotten the message that its 2008 EHS strategic plan is inadequate. Efforts are under way to revise that plan, and for the first time, the ethical, legal, and social implications of nanotechnology are being considered.
NNI is also planning to rewrite its strategic plan for the entire program, not just EHS research, according to Sally S. Tinkle, who cochairs the National Science & Technology Council’s Nanoscale Science, Engineering & Technology Subcommittee. That subcommittee is responsible for coordinating the development of NNI’s strategic plan and mechanisms for interagency communication and coordination on nanotech R&D.
Advertisement
At the March workshop, Tinkle emphasized that NNI will integrate EHS studies into all of its research.
“EHS considerations have to be a part of all of our work in nanotechnology,” echoed Jeff Morris, national program director for nanotechnology in EPA’s Office of Research & Development. The ultimate success will come “when EHS research is no longer considered a separate area, but rather an integral part of all we do as we advance nanotechnology,” he said.



Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter