Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Biological Chemistry
Slipping In An Acetyl For Enzyme Control
Controlled acetylation inhibits isomerase activity of cyclophilin A, which could lead to new antiviral treatments
by Sarah Everts
April 12, 2010
| A version of this story appeared in
Volume 88, Issue 15
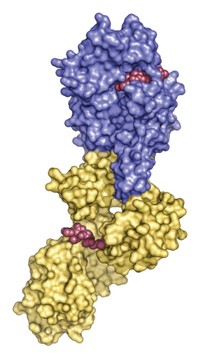
Acetylation of proteins helps biological cells control a number of processes, including glycolysis and DNA repair. But the inability to install an acetyl group on a specific lysine residue in the lab has stymied biochemical studies of this posttranslational modification. A team led by Leo C. James and Jason W. Chin of the Medical Research Council Laboratory of Molecular Biology, in Cambridge, England, has used a method previously created by Chin’s group to help rectify this problem. The researchers produced cyclophilin A (CypA) proteins in Escherichia coli that have acetyl groups installed at a targeted lysine residue (Nat. Chem. Biol., DOI: 10.1038/nchembio.342). They found that this particular acetylation, located in the active site of CypA, blocks the enzyme’s ability to perform cis-trans isomerization of peptide bonds next to proline residues. CypA is thought to help HIV-1 infection by isomerizing the viral capsid and triggering disassembly, James notes. Because acetylation inhibits CypA cis-trans catalysis, the team predicts it would inhibit disassembly and hinder infection and could one day be the focus of antiviral treatments.


Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter