Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Materials
Crystalline Sheets, Courtesy Of A Cosolvent
Adding a chloroalkane to the preparation of PbS nanocrystals shifts the crystal shape from spheres to ultrathin sheets
by Bethany Halford
August 2, 2010
| A version of this story appeared in
Volume 88, Issue 31
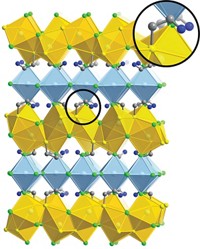
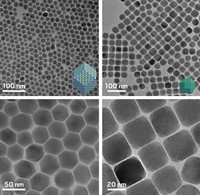
By adding a chloroalkane cosolvent to the standard procedure for preparing lead sulfide nanocrystals, chemists have shifted the shape the crystals take from spheres to ultrathin sheets (Science 2010, 329, 550). The quality of the resulting sheets, which are several hundred nanometers across but only a few nanometers thick, is so high that the material can be integrated into a photodetector without any further processing. The research comes from a group led by Horst Weller of the University of Hamburg, in Germany. According to the researchers, the chlorinated solvent encourages two-dimensional sheet growth over 3-D particle formation by slowing down the PbS growth rate during primary nanocrystal formation. This process, in turn, exposes highly reactive facets on the growing crystal surface that act as fusing points for other nanocrystals. A layer of oleic acid—an organic ligand used in the nanoparticle synthesis—forms on the sheet’s surfaces, driving the 2-D growth. This so-called oriented attachment of nanocrystal building blocks “is one of the most promising approaches in nanotechnology,” the researchers note.



Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter